Abstract
Background
The use of surgery in patients with locally advanced pancreatic cancer (LAPC) following induction chemotherapy is increasing. However, most series do not report on the total cohort of patients undergoing surgical exploration; therefore, this single-center study investigates outcomes among all consecutive patients with LAPC who underwent surgical exploration.
Methods
We conducted a retrospective, single-center analysis including all consecutive patients with LAPC (Dutch Pancreatic Cancer Group criteria) who underwent surgical exploration with curative intent (January 2014–June 2023) after induction therapy. Primary outcomes were resection rate and overall survival (OS) from the time of diagnosis.
Results
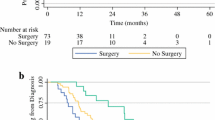
Overall, 127 patients underwent surgical exploration for LAPC, whereby 100 patients (78.7%) underwent resection and 27 patients (21.3%) underwent a non-therapeutic laparotomy due to the extent of vascular involvement (n = 11, 8.7%) or occult metastases (n = 16, 12.6%). The overall in-hospital/30-day mortality rate was 0.8% and major morbidity was 31.3% (in patients after resection: 1.0% and 33.3%, respectively). The overall 90-day mortality rate was 5.5%, which included 3.1% mortality due to disease progression. Resection was associated with longer median OS {29 months (95% confidence interval [CI] 26–43) vs. 17 months (95% CI 11–26); p < 0.001} compared with patients undergoing non-therapeutic laparotomy, with corresponding 5-year OS rates of 28.4% and 7.7%. In Cox proportional hazard regression analysis, only pancreatic body/tail tumors independently predicted OS (hazard ratio 1.788 [95% CI 1.042–3.068]).
Conclusion
This single-center series found a resection rate of 78.7% in patients with LAPC selected for surgical exploration, with a low risk of mortality and morbidity in all explored patients and a 5-year OS rate after resection of 28.4%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Approximately 30–40% of patients with pancreatic adenocarcinoma are diagnosed with locally advanced pancreatic cancer (LAPC) due to extensive vascular tumor involvement. Upfront surgery in these patients is precluded because of high surgical risks in the absence of survival benefit;1,2 therefore, patients with LAPC have historically been treated with non-curative intent, comprising either palliative chemotherapy or best supportive care.
Over the last decade, the treatment for LAPC strongly evolved due to the introduction of novel multiagent chemotherapy regimens (i.e., [m]FOLFIRINOX and gemcitabine-nab-paclitaxel), which are associated with improved disease control, resection rates, and increased overall survival (OS).3,4 International expert centers have gradually improved their selection process for surgical resection of LAPC,5 using anatomical, biological and conditional parameters.6,7 As a consequence, approximately 19–22% of selected patients with LAPC undergo resection, associated with a median OS of 28–33 months.8,9
Regardless of improved induction therapy regimens and adequate response evaluation, a considerable subgroup of surgically explored patients with LAPC will undergo a non-therapeutic laparotomy without resection, due to either extensive local involvement or radiological occult metastases.10 However, most series do not report the rate of non-therapeutic laparotomy in this setting, let alone the postoperative outcome in this subgroup.11,12,13,14,15,16,17 Moreover, studies reporting on predictors for surgical and oncological outcomes in a cohort of explored LAPC patients within an homogenous, high-volume center, which may guide and optimize patient selection for surgery, are currently lacking.
Therefore, this study sought to investigate postoperative outcome and predictors for OS among all consecutive patients with LAPC who underwent surgical exploration with intention for resection in a single high-volume center in The Netherlands.
Methods
Study Design
This single-center, retrospective study was performed following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.18 Data were extracted from the mandatory prospective Dutch Pancreatic Cancer Audit,19 whereby additional data were obtained through the prospectively maintained nationwide LAPC Registry.20 Missing data were collected from local electronic medical records.
Study Population
All consecutive patients (≥18 years of age) with LAPC at diagnosis who underwent a surgical exploration with intention for a resection following systemic induction chemotherapy in the Amsterdam UMC were included (1 January 2014 to 30 June 2023). Patients with (borderline) resectable disease who progressed to LAPC during neoadjuvant chemoradiotherapy were also included, as long as they thereafter received a second-line induction therapy for LAPC.
Outcomes
The primary outcome was resection rate and OS from the time of diagnosis (i.e., imaging-based and pathology confirmed). Secondary outcomes included pre- and intraoperative predictors for OS in the overall cohort as well as in-hospital/30-day mortality and major morbidity, and 90-day mortality.
Definitions
LAPC was defined in accordance with the Dutch Pancreatic Cancer Group (DPCG) criteria: >90° arterial involvement (i.e., superior mesenteric artery, celiac axis, and/or any hepatic artery) and/or >270° portomesenteric venous involvement.21 In the case of occlusion or non-reconstructable portomesenteric venous involvement, patients were also classified as LAPC, as outlined in electronic supplementary Table 1.21 Additionally, LAPC was also defined in accordance with the 2022 National Comprehensive Cancer Network (NCCN) guideline.22 Further details regarding methodological definitions are described in Appendix 1.
Patient Selection
From January 2014 to May 2021, surgical procedures were performed at location AMC of Amsterdam UMC. Thereafter, following a hospital merger, all procedures were performed at location VUmc of Amsterdam UMC (June 2021–June 2023). In general, patients with non-progressive disease (Response Evaluation Criteria in Solid Tumors [RECIST] criteria)23 on four-phase computed tomography (CT) scan combined with serum tumor marker response following at least 2 months of systemic chemotherapy (either four cycles of [m]FOLFIRINOX or two cycles of gemcitabine-nab-paclitaxel) were selected for surgery during a multidisciplinary team meeting. Serum carbohydrate antigen 19-9 (CA19-9) and, since 2021, fluorodeoxyglucose positron emission tomography with CT (FDG-PET/CT) were used to determine the biological tumor response to induction therapy.24 In January 2022, the nationwide PREOPANC-4 trial started in the Amsterdam UMC (ClinicalTrials.gov identifier: NCT05524090), wherein the international best-practice for LAPC care is implemented in The Netherlands, in close collaboration with four international experts.25 As a consequence, the institutional treatment protocol for patients with LAPC changed. First, the induction phase was extended from at least 2 months to a minimum of 4 months of chemotherapy (i.e., eight cycles [m]FOLFIRINOX/four cycles gemcitabine-nab-paclitaxel), whereby a chemotherapy switch (i.e., second-line therapy) was considered in case of biological and/or radiological (local) disease progression.
Statistical Analyses
Data analyses were performed using RStudio: Integrated Development Environment for R (software version 1.3.1093, Boston, MA, USA).26 Descriptive statistics were compiled to summarize patient characteristics. Pearson’s Chi-square, or Fisher’s exact test when appropriate, was used to compare categorical variables, and the Mann–Whitney U test was used to compare numerical variables. The Cochran–Armitage Test was used to assess trends over time. Categorical variables are presented as percentages and frequencies, and numerical variables as medians with corresponding interquartile ranges (IQRs). For all tests, statistical significance was defined as a two-tailed p-value of <0.050. Recurrence-free survival was calculated from the date of surgery, and OS was calculated from the date of diagnosis to the date of death or last follow-up, and presented using Kaplan–Meier estimates with their corresponding 95% confidence intervals (CIs). Both were assessed using the log-rank test. Patients still alive during the date of final follow-up (31 October 2023) were censored. Median follow-up was calculated through reversed Kaplan–Meier estimates.
Cox proportional hazards regression analysis was performed to identify potential predictors for OS. Results of the regression analysis are presented as hazard ratios (HR) with corresponding 95% CIs. Clinical predictors with a p-value <0.200 in univariable analysis were included in the multivariable analysis. Backwards stepwise selection was used for the removal of insignificant variables in multivariable analysis, until all remaining variables were statistically significant.27 In order to correct for implementation of the international best practice for LAPC in our center (including the training program from 2021 to 2022), a categorical variable was added in multivariable analysis distinguishing patients treated between 2014–2020 and 2021–2023. The relative serum CA19-9 response following induction therapy was categorized according to the optimal response cut-off value for LAPC, as determined previously.28
Preferably, imputation was performed for the missing CA19-9 data. However, a broad variety existed between the imputed datasets and the original dataset that could have resulted in misinterpretation of outcomes; therefore, no imputation was performed and an additional ‘missing’ category was added for each categorical variable that was missing ≥2%. Patients with missing data for variables missing <2% were excluded. If the only significant level in a variable was the ‘missing’ category, this independent variable was not included in the multivariable analysis.
Results
During the study period, 127 patients with LAPC were selected for surgical exploration with intention for resection and were subsequently included in this study. All patients received systemic induction chemotherapy, which mostly consisted of (m)FOLFIRINOX (n = 117, 92.1%) with a median of four cycles (4–8), whereby eight patients (6.3%) were treated with second-line induction chemotherapy prior to surgery. In total, four patients (3.1%) who were initially diagnosed with borderline resectable disease who progressed to LAPC during neoadjuvant therapy were included (see Table 1 for patient and treatment characteristics).
Response to Induction Therapy
The majority of patients (n = 78, 75.7%) presented with RECIST stable disease on imaging following systemic induction therapy, while the remaining patients presented with either partial response (n = 24, 23.3%) or local progressive disease (n = 1, 1.0%) at restaging. This latter patient arbitrarily underwent surgery because of stable non-elevated serum CA19-9 during chemotherapy and no change in vascular involvement, although tumor size increased.
At restaging, the median serum CA19-9 level was 62 U/mL (IQR 23–186), including normalization in 17 patients (22.1%) among those with elevated CA19-9 prior to induction therapy. No significant differences were observed in CA19-9 response to induction therapy between those resected and those undergoing non-therapeutic laparotomy. However, patients not undergoing resection more often had non-elevated (<37 U/mL) serum CA19-9 levels at restaging (30.4% vs. 51.9%; p = 0.004) [see electronic supplementary Table 2 for anatomical and biological tumor response to induction therapy].
Surgical Procedures and Outcome
Diagnostic laparoscopy was performed in 101 patients (79.9%) at the start of surgical exploration, whereby an annual increase was seen from 16.7% in 2014 to 98.4% in 2022 (p < 0.001). Overall, 21.3% (n = 27) of all patients did not undergo tumor resection due to the extent of vascular involvement (n = 11/127, 8.7%) or metastatic disease (n = 16/127, 12.6%). These latter 16 patients had either radiographic occult metastases detected during laparoscopy (n = 6/78, 7.8%) or during surgical exploration (n = 10/127, 7.8%). The remaining 100 patients (78.7%) underwent pancreatic resection, mostly pancreatoduodenectomy (n = 77/100, 77.0%). Vascular resections were performed in 68 patients (68.0%), including portomesenteric venous resection (n = 57/100, 57.0%) and/or arterial resection[s] (n = 17/100, 17.0%). Multivisceral resection was performed in nine patients (9.0%). Procedural details are summarized in Table 2. The resection rates for patients with NCCN LAPC (n = 36/49, 73.5%) at initial diagnosis did not differ from the resection rates in the remaining patients (n = 64/78, 82.1%; p = 0.250).
The rates of in-hospital/30-day mortality and major morbidity were 0.8% and 31.3%, respectively. No significant differences were seen for patients with or without resection in in-hospital/30-day mortality (1.0% vs. 0%; p = 0.999) and major morbidity (33.3% vs. 25.9%; p = 0.464), respectively. The 90-day mortality rate was 5.5%, which included 3.1% mortality due to early disease progression (in patients after resection this was 5.0% and 2.0%, respectively). Among patients who underwent resection, the R1 resection rate was 53.0% (n = 53) with pN0 resection in 36.0% (n = 36). In total, 61 patients (61.0%) received adjuvant therapy following resection (see Table 3 for details regarding surgical and histopathological outcome for both groups).
Oncological Outcome
The median follow-up time from time of diagnosis in all patients was 27 months (IQR 22–29), whereby 70 patients (55.1%) were alive at the end of follow-up. The median OS was 28 months from the time of diagnosis (95% CI 24–34) and 23 months (95% CI 17-30) from the time of surgery. In the full cohort of 127 patients, the 1-, 3-, and 5- year OS rates from date of diagnosis were 84.6%, 33.2%, and 23.7%, respectively. Resection was associated with a longer median OS as compared with patients undergoing non-therapeutic laparotomy (29 months [95% CI 26-43] vs. 17 months [95% CI 11–26]; p < 0.001), as illustrated in Fig. 1. The 1-, 3-, and 5-year OS rates were 88.3%, 37.8%, and 28.4% in patients who underwent resection, respectively, versus 69.5%, 15.4%, and 7.7% in patients undergoing non-therapeutic laparotomy, respectively. The 5-year OS rates in patients with NCCN LAPC were 30.5% in those resected and 13.6% in those undergoing non-therapeutic laparotomy. In sensitivity analysis, all patients undergoing local ablative therapy (n = 6) were excluded from the non-therapeutic laparotomy group, resulting in a median OS of 14 months (95% CI 10–19) in the remaining 21 patients.
Disease recurrence was observed in 62% of patients after resection (n = 62/100). Most patients developed both locoregional recurrence and distant metastases (n = 31/64, 48.4%). Only local recurrence occurred in 18.8% (n = 12/64) and only distant metastases occurred in 30.6% (n = 19/64) of patients. The median recurrence-free survival was 10 months (95% CI 9–13), whereby 24 patients (24.0%) presented with recurrence at ≤6 months (early recurrence) and 21 patients (21.0%) presented with recurrence at ≥12 months (late recurrence).
In the overall cohort, Cox proportional hazard regression analysis only identified tumors located in the pancreatic body/tail as an independent negative predictor for OS (HR 1.788 [95% CI 1.042–3.068]) [see Table 4]. In patients undergoing resection, only the presence of positive lymph nodes at pathological assessment (ypN1-2) was identified as a negative predictor for OS (HR 3.503 [95% CI 1.630–7.527]) [see electronic supplementary Table 3 for the Cox regression analysis for resected patients].
Trend in Surgical Exploration
The annual number of patients with LAPC undergoing surgical exploration increased over time from 6 in 2014 to 19 in 2022 (p = 0.543). No trend was seen in the number of non-therapeutic laparotomies over time (either absolute or relative to the total number of explorations), as outlined in electronic supplementary Fig. 1.
Discussion
This third largest single-center experience demonstrated a 78.8% resection rate among 127 patients with LAPC selected for surgical exploration after induction chemotherapy. The in-hospital/30-day mortality was 0.8% and median OS after resection was 29 months. In the 27 patients in whom no resection was performed, 59.2% had occult metastatic disease. Median OS was significantly longer for patients undergoing resection, with a 5-year OS rate of 28.4% versus 7.7% in patients in whom no resection was performed. During the study period, the use of surgery for patients with LAPC gradually increased.
Previously, several high-volume centers from the United States, Germany, Sweden, Italy, France, and Japan have reported their experience with surgical resection for LAPC following induction chemotherapy.11,12,13,14,15,16,17,29 One study did not distinguish outcomes for patients with locally advanced or metastatic disease,13 and the remaining studies are summarized in Table 5. The majority of studies included both borderline resectable pancreatic cancer (BRPC) and LAPC as defined by the NCCN guideline,11,12,13,14,15,16,17 which is comparable with the DPCG definition for LAPC in the current study, as outlined in electronic supplementary Table 1. Of these, only one study previously reported on the surgical and oncological outcomes of all explored patients, and subsequently on outcomes of the non-therapeutic laparotomy group only.29 Herein, Michelakos and colleagues reported on 141 patients with BRPC/LAPC undergoing surgical exploration after (m)FOLFIRINOX with a similar resection rate (78.7% vs. 78.0%) and median OS after non-therapeutic laparotomy (17 vs. 19 months).29 However, potential predictors for OS in the total cohort were not investigated. A second study only reported on 3-year OS after non-therapeutic laparotomy (2.4%), which was considerably shorter than the 15.4% in the current study. The reasons for this difference are yet unclear.
The remaining six studies only reported on outcomes in the resected patients. The in-hospital/30-day mortality and 90-day mortality rates after resection were comparable with those reported in the current study (0–6% vs. 1%)13,15,16,17,29 and (4–8% vs. 5%), respectively.11,12,16,17,30 Likewise, median OS from diagnosis (15–51 vs. 29 months)11,12,13,16,17,29 and actuarial 5-year OS (16–33% vs. 28%)11,17,31 following resection as described are in line with the current study.
Historically, there has been considerable reluctance towards surgical exploration in patients with LAPC.32 Despite 5-year OS rates reported up to 33%, recurrence occurs in more than 80% of patients and remains the principal cause of limited survival benefit following surgery.33 In particular, early recurrence (≤6 months) remains a major problem, as it occurs in one-third of patients after surgery for LAPC and is associated with decreased OS compared with patients with late recurrence (≥12 months) (8.4 vs. 27.4 months). This finding suggests the presence of occult systemic disease at the time of surgery.34 In this study, 62% of resected patients developed recurrence before the end of follow-up, associated with a median recurrence-free survival of 10 months. Additionally, approximately one-quarter of patients developed early recurrence, in whom surgery may not have been effective.
The inability to accurately predict patients at risk for (early) recurrence in combination with a non-therapeutic laparotomy rate of 21% underlines the persisting complexities in patient selection for surgery.35 Particularly in the current era of multiagent induction therapy, which is known to hamper the predictive value of CT-based restaging,36 the concept of tumor biology-driven response evaluation has become crucial in patient selection.6 This mainly relies on serum CA19-9 and, to a lesser extent, functional imaging modalities determining the metabolic and biological response to induction therapy.37 FDG-PET/CT could support decision making based on tumor markers as serum CA19-9, although more data are needed.37
In order to optimize patient stratification and surgical decision making in LAPC, numerous predictors for OS have been described, including sex, age, tumor diameter, vascular involvement, tumor markers, tumor differentiation, and length of preoperative therapy.38,39,40 Somewhat unexpected, besides tumor location, no preoperative predictors useful for patient selection were identified in the overall cohort in the current study. Serum CA19-9 levels at restaging, both as absolute values and categorized by response, were not identified as independent predictors for OS. This is likely due to the fact that patients included in the Cox regression analysis were, among others, selected to undergo surgical exploration based on biological response of CA19-9 following induction therapy. Predictors for OS described in the literature are often derived from an unselected cohort including all LAPC patients receiving systemic therapy, and hampered by the inclusion of tumor resection (yes/no) as an independent variable.39,40 A previous Dutch nationwide study identified age, sex, comorbidity status, and serum CA19-9 levels as independent predictors for OS, while only including preoperative parameters that may aid in patient selection.38 Nevertheless, the vast majority of the cohort comprised patients who did not undergo resection (n = 220/252). Among surgical candidates in patients with LAPC, predictors identified in an homogeneous cohort of patients with LAPC explored with curative intent may be of value in preoperative clinical decision making and, in particular, the prevention of non-therapeutic laparotomies and futile surgical resection by early disease recurrences. To the best of the authors knowledge, no study has reported on predictors identified in an overall cohort of patients undergoing surgical exploration for LAPC (i.e., with and without resection).
Besides preoperative selection, accurate intraoperative staging may prevent morbidity and loss of quality of life from futile operation. Nevertheless, 21% of explored patients underwent a non-therapeutic laparotomy in the current study, which is rather similar to the 28% reported by a recent multicenter study comprising five international expert centers, including our center.10 Diagnostic laparoscopy can aid in the detection of occult metastatic disease. In our center, patients undergoing surgical exploration for any stage of pancreatic ductal adenocarcinoma undergo laparoscopy in the same procedure as the intended laparotomy. However, lack of a standardized approach towards the use of laparoscopy in the early years of the study period resulted in only 79.9% of patients undergoing laparoscopy, which identified metastases in 7.8% of these patients. This is underlined by the increase seen in the use of laparoscopy from 16.7% in 2014 to 98.4% in 2022 (p < 0.001). Moreover, the reasons for incomplete laparoscopy (e.g. adhesions) were not registered and these data are therefore lacking. A previous retrospective study including 151 patients in whom occult metastatic disease was detected either by diagnostic laparoscopy (n = 89) or laparotomy (n = 62) found better OS if detected by laparoscopy (343 vs. 222 days), underlining its value.41 Interestingly, recent data from the North American National Surgical Quality Improvement Program (NSQIP) registry reported on a decreasing trend for the utilization of diagnostic laparoscopy in an 8-year study period, whereby only 10% of patients underwent staging laparoscopy (for all stages of localized pancreatic cancer).42 This is contradictory to the practice reported from several international expert centers and in the current study.43,44,45
In order to optimize oncological treatment strategies and subsequent patient selection for surgery in LAPC, the nationwide PREOPANC-4 trial was started in 2021 (ClinicalTrials.gov identifier: NCT05524090), implementing the international best practices for LAPC care in The Netherlands.25 In close collaboration with four international expert centers from the United States and Germany, multidisciplinary care and selection for surgery is currently being optimized by standardization of oncological treatments and patient selection for surgery. The rather similar 5-year OS rates in patients with NCCN LAPC compared with the overall cohort (30% vs. 28%) in combination with similar resection rates (73.5% vs. 82.1%; p = 0.250) illustrate the ongoing paradigm shift from anatomical-driven towards biology-driven patient selection for surgery. Future research should focus on improving response evaluation to induction therapy for surgery by the development of more personalized treatment and the identification and validation of new serological/imaging-based tumor markers. Molecular and genomic profiling could help to predict tumor sensitivity to induction therapy.46,47 Additionally, a wide spectrum of promising biomarkers have been described, such as inflammatory response parameters, circulating tumor DNA and microRNAs, which may help in patient selection.48,49 Nevertheless, it is important that their added value against CA19-9 is confirmed.50
Conclusion
This high-volume, single-center analysis found a 78.7% resection rate in 127 selected patients with LAPC who underwent surgical exploration. Resection was associated with a median OS of 29 months and a 5-year OS rate of 28.4%, whereas the median OS and 5-year OS among 27 patients undergoing non-therapeutic laparotomy were 17 months and 7.7%, respectively. In the overall cohort, only tumor location was associated with OS. As predictors for OS to guide pre- and intraoperative decision making remain limited, future research should focus on optimizing the selection criteria for surgery in LAPC patients by identification and validation of tumor markers aimed at preventing futile operation.
References
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–20.
Oba A, Ho F, Bao OR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020;10:245.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Stoop TF, Theijse RT, Seelen LWF. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21(2):101–24.
Oba A, Del Chiaro M, Fujii T, et al. “Conversion surgery” for locally advanced pancreatic cancer: a position paper by the study group at the joint meeting of the international association of pancreatology (IAP) & Japan pancreas society (JPS) 2022. Pancreatology. 2023;23(6):712–20.
Oba A, Del Chiaro M, Satoi S, et al. New criteria of resectability for pancreatic cancer: a position paper by the Japanese society of hepato-biliary-pancreatic surgery (JSHBPS). J Hepatobiliary Pancreat Sci. 2022;29(7):725–31.
Brown ZJ, Heh V, Labiner HE, et al. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: meta-analysis. Br J Surg. 2022;110:34–42.
Eshmuminov D, Aminjonov B, Palm RF, et al. FOLFIRINOX or gemcitabine-based chemotherapy for borderline resectable and locally advanced pancreatic cancer: a multi-institutional, patient-level, meta-analysis and systematic review. Ann Surg Oncol. 2023;30(7):4417–28.
Theijse RT, Stoop TF, Janssen QP, et al. Impact of a non-therapeutic laparotomy in patients with locally advanced pancreatic cancer treated with induction (m)FOLFIRINOX: a TAPS consortium study. Br J Surg. 2024;111(3):znae033.
Rangelova E, Wefer A, Persson S, et al. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg. 2021;273(3):579–86.
Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273(2):341–9.
Hackert T, Sachsenmaier M, Hinz U, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457–63.
Maggino L, Malleo G, Marchegiani G, et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 2019;154(10):932–42.
Ushida Y, Inoue Y, Oba A, et al. Optimizing indications for conversion surgery based on analysis of 454 consecutive Japanese cases with unresectable pancreatic cancer who received modified FOLFIRINOX or gemcitabine plus nab-paclitaxel: a single-center retrospective study. Ann Surg Oncol. 2022;29(8):5038–50.
Gemenetzis G, Groot VP, Blair AB, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 2019;70(2):340–7.
Garnier J, Ewald J, Marchese U, et al. Borderline or locally advanced pancreatic adenocarcinoma: a single center experience on the FOLFIRINOX induction regimen. Eur J Surg Oncol. 2020;46(8):1510–5.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
van Rijssen LB, Groot Koerkamp B, Zwart MJ, et al. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the Dutch pancreatic cancer audit. HPB. 2017;19(10):919–26.
Walma MS, Brada LJ, Patuleia SIS, et al. Treatment strategies and clinical outcomes in consecutive patients with locally advanced pancreatic cancer: a multicenter prospective cohort. Eur J Surg Oncol. 2021;20:699–707.
Dutch Pancreatic Cancer Group. DPCG-definities resectabiliteit pancreascarcinoom. Dutch Pancreatic Cancer Group; 2012.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 1.2022, NCCN clinical practice guidelines in oncology. National comprehensive cancer network. 11 Apr 2022. Available at: https://www.nccn.org/guidelines/guidlines-detail?category=1&id=1455. Accessed 11 Apr 2022.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Soloff E, Al-Hawary M, Desser TS, Fishman EK, Minter RM, Zins M. Imaging assessment of pancreatic cancer resectability after neoadjuvant therapy: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218(4):570–81.
Stoop TF, Seelen LWF, van't Land FR, et al. PREOPANC4 implementation program for locally advanced pancreatic cancer (PREOPANC-4). 1 Sep 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05524090?term=PREOPANC4&draw=2&rank=1. Accessed 3 Sep 2022.
RStudio: Integrated development for R. RStudio, PBC, Boston. (2020).
Sperandei S. Understanding logistic regression analysis. Biochem Med. 2014;24(1):12–8.
Seelen LWF, Doppenberg D, Stoop TF, et al. Minimum and optimal CA19-9 response after two months induction chemotherapy in patients with locally advanced pancreatic cancer: a nationwide multicenter study. Ann Surg. 2024;279(5):832–41. https://doi.org/10.1097/sla.0000000000006021.
Michelakos T, Pergolini I, Castillo CF, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269(4):733–40.
Weniger M, Moir J, Damm M, et al. Respect: a multicenter retrospective study on preoperative chemotherapy in locally advanced and borderline resectable pancreatic cancer. Pancreatology. 2020;20(6):1131–8.
McIntyre CA, Cohen NA, Goldman DA, et al. Induction FOLFIRINOX for patients with locally unresectable pancreatic ductal adenocarcinoma. J Surg Oncol. 2022;125(3):425–36.
Schneider M, Hackert T, Strobel O, Büchler MW. Technical advances in surgery for pancreatic cancer. Br J Surg. 2021;108(7):777–85.
Groot VP, Blair AB, Gemenetzis G, et al. Recurrence after neoadjuvant therapy and resection of borderline resectable and locally advanced pancreatic cancer. Eur J Surg Oncol. 2019;45(9):1674–83.
Seelen LW, Van Oosten AF, Brada LJ, Groot VP, Daamen LA, Walma MS, Van Der Lek BF, Liem MS, Patijn GA, Stommel MW, Van Dam RM. Early recurrence after resection of locally advanced pancreatic cancer following induction therapy: an international multicenter study. Ann Surg. 2023;278(1):118–26.
Wu YHA, Oba A, Lin R, Watanabe S, Meguid C, Schulick RD, Del Chiaro M. Selecting surgical candidates with locally advanced pancreatic cancer: a review for modern pancreatology. J Gastrointest Oncol. 2021;12(5):2475–83.
Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12–7.
Ghidini M, Vuozzo M, Galassi B, et al. The role of positron emission tomography/computed tomography (PET/CT) for staging and disease response assessment in localized and locally advanced pancreatic cancer. Cancers. 2021;13(16):4155.
Brada LJH, Walma MS, Daamen LA, et al. Pedicting oveall survival and resection in patients with locally advanced pancreatic cancer treated with FOLFIINOX: development and internal validation of two nomograms. J Surg Oncol. 2021;124(4):589–97.
Bednar F, Zenati MS, Steve J, et al. Analysis of predictors of resection and survival in locally advanced stage III pancreatic cancer: does the nature of chemotherapy regimen influence outcomes? Ann Surg Oncol. 2017;24:1406–13.
Lu Z, Shao W, Shi X, et al. Identifying optimal candidates for tumor resection among borderline and locally advanced pancreatic cancer: a population-based predictive model. Pancreatology. 2022;22:286–93.
Sell NM, Fong ZV, Del Castillo CF, et al. Staging laparoscopy not only saves patients an incision, but may also help them live longer. Ann Surg Oncol. 2018;25(4):1009–16.
Paracha M, Van Orden K, Patts G, Tseng J, McAneny D, Sachs T. Opportunity lost? diagnostic laparoscopy in patients with pancreatic cancer in the national surgical quality improvement program database. World J Surg. 2019;43(3):937–43.
Fong ZV, Alvino DML, Fernández-Del Castillo C, et al. Reappraisal of staging laparoscopy for patients with pancreatic adenocarcinoma: a contemporary analysis of 1001 patients. Ann Surg Oncol. 2017;24:3203–11.
Oba A, Inoue Y, Ono Y, et al. Staging laparoscopy for pancreatic cancer using intraoperative ultrasonography and fluoresence imaging: the SLING trial. Br J Surg. 2021;108(2):115–8.
Gudmundsdottir H, Yonkus JA, Alva-Ruiz R, et al. Yield of staging laparoscopy for pancreatic cancer in the modern era: analysis of more than 1,000 consecutive patients. J Am Coll Surg. 2023;237(1):49–57.
Sivapalan L, Kocher HM, Ross-Adams H, Chelala C. The molecular landscape of pancreatic ductal adenocarcinoma. Pancreatology. 2022;22(7):925–36.
Aung KL, Fischer SE, Denroche RE, et al. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin Cancer Res. 2018;24(6):1344–54.
Kinny-Köster B, Habib J, Wolfgang CL, He J, Javed AA. Favorable tumor biology in locally advanced pancreatic cancer—beyond CA19-9. J Gastrointest Oncol. 2021;12:2484–94.
Ueberroth BE, Jones JC, Bekaii-Saab TS. Circulating tumor DNA (ctDNA) to evaluate minimal residual disease (MRD), treatment response, and posttreatment prognosis in pancreatic adenocarcinoma. Pancreatology. 2022;22(6):741–8.
Boyd LNC, Ali M, Leeflang MMG, et al. Diagnostic accuracy and added value of blood-based protein biomarkers for pancreatic cancer: a meta-analysis of aggregate and individual participant data. EClinicalMedicine. 2023;55:101747.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
Johanna W. Wilmink has received grants from Servier and MSD; payment or honoraria for lectures and presentations from Servier and MSD/Merck; and held Advisory Board roles for Astra Zeneca, Servier, and MSD. Rutger T. Theijse, Thomas F. Stoop, Philip D. Leenart, Kishan R.D. Lutchman, Joris I. Erdmann, Freek Daams, Babs M. Zonderhuis, Sebastiaan Festen, Rutger-Jan Swijnenburg, Thomas M. van Gulik, Annuska Schoorlemmer, André L.A. Sterk, Susan van Dieren, Arantza Fariña, Rogier P. Voermans, Geert Kazemier, Olivier R. Busch, and Marc G. Besselink have no disclosures to declare in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rutger T. Theijse and Thomas F. Stoop are co-first authors on this work.
Olivier R. Busch and Marc G. Besselink are co-senior authors on this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Theijse, R.T., Stoop, T.F., Leenart, P.D. et al. Surgery for Locally Advanced Pancreatic Cancer Following Induction Chemotherapy: A Single-Center Experience. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15591-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15591-4