Abstract
Background
Traumatic brain injury (TBI) causes mortality and long-term disability among young adults and imposes a notable cost on the healthcare system. In addition to the first physical hit, secondary injury, which is associated with increased intracranial pressure (ICP), is defined as biochemical, cellular, and physiological changes after the physical injury. Mannitol and Hypertonic saline (HTS) are the treatment bases for elevated ICP in TBI. This systematic review and meta-analysis evaluates the effectiveness of HTS in the management of patients with TBI.
Methods
This study was conducted following the Joanna Briggs Institute (JBI) methods and PRISMA statement. A systematic search was performed through six databases in February 2022, to find studies that evaluated the effects of HTS, on increased ICP. Meta-analysis was performed using comprehensive meta-analysis (CMA).
Results
Out of 1321 results, 8 studies were included in the systematic review, and 3 of them were included in the quantitative synthesis. The results of the meta-analysis reached a 35.9% (95% CI 15.0–56.9) reduction in ICP in TBI patients receiving HTS, with no significant risk of publication bias (t-value = 0.38, df = 2, p-value = 0.73). The most common source of bias in our included studies was the transparency of blinding methods for both patients and outcome assessors.
Conclusion
HTS can significantly reduce the ICP, which may prevent secondary injury. Also, based on the available evidence, HTS has relatively similar efficacy to Mannitol, which is considered the gold standard therapy for TBI, in boosting patients' neurological condition and reducing mortality rates.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is the most common cause of mortality and long-term disability among young adults [1]. It is estimated that about seventy million individuals suffer from TBI each year [2], which makes it a consequential public health concern worldwide and imposes a significant cost on the healthcare system [3]. Road traffic injuries, falls, and violence are among the most common cause of TBI [4].
The first physical hit is not the only injurious mechanism in TBI. Biochemical, cellular, and physiological changes after the physical injury, which is called secondary injury [5], are significantly associated with poor neurological outcomes and mortality in these patients [6]. Studies suggested that increased intracranial pressure (ICP), which is a common complication associated with TBI [7], is a factor associated with secondary injury in TBI patients [8].
Hyperosmolar therapy, such as Mannitol and Hypertonic saline (HTS), is one of the primary treatment bases for elevated ICP in TBI [9, 10]. Several insights have been gained about HTS and mannitol in TBI. HTS is considered routine care in TBI patients [11]. Several studies assessed the efficacy of hyperosmolar components in decreasing ICP and overall outcomes of patients with TBI, but there are still controversies in this regard [12, 13]. In a meta-analysis of randomized controlled trials (RCTs) in 2016, HTS was compared with any other solutions in severe TBI. This study found no significant difference between HTS and other solutions in lowering mortality or improving ICP [14]. Also, a recent Cochrane review in 2020, based on weak available evidence, found that HTS is no better than mannitol in TBI patients [15].
These mentioned reviews did not include recent publications. In addition, observational studies were not included in these studies. This systematic review and meta-analysis evaluates the efficacy of HTS in the management of elevated ICP secondary to TBI, as the primary outcome. The effects of HTS in lowering mortality rates and improving neurological outcomes are also investigated as secondary outcomes.
Methods
This systematic review was completed following the methods reported in Joanna Briggs Institute (JBI) Manual for Evidence Synthesis [16] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17].
Eligibility criteria
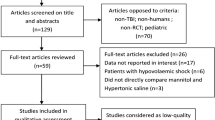
Studies, that assessed the effects of HTS in any concentration and dosage, on ICP in patients with TBI were included in this systematic review. Non-English papers, review articles, commentaries, letters, and these were not included.
Search
A systematic search was conducted in Medline via PubMed, EMBASE, Scopus, ProQuest, Google Scholar, and Web of Science in February 2022 with no limitations. The details of search strategies are presented in Additional file 1.
Study selection
The results of database searches were imported into EndNote × 9 software and after removing the duplicated results, two independent researchers (NG, SD) assessed the meeting eligibility criteria in two title/abstract and full-text stages. Disagreements in the study selection process were resolved through consultation or by referring to another author (HS), who is an expert in this topic.
Data collection
Data extraction was conducted using an electronic table in Microsoft excel which included the following parameters: the name of the first author of the study, the publication year, study design, setting of the study, mean age of the participants, the male ratio, assessed interventions, mortality rate, neurological outcomes, and outcomes about ICP.
Risk of bias assessment
The risk of bias in RCTs was assessed using the JBI checklist [18]. JBI critical appraisal tool for RCTs assesses the risk of bias regarding the randomization, allocation concealment, the similarity of the groups in the baseline, blinding of participants and researchers, identic received treatment (other than the intervention of interest), follow-up completion, analyzing the participants in the groups to which they were randomized, identic and reliable outcomes measurement, and statistical analysis. For cross-sectional studies [19], quasi-experimental studies [18], and case–control studies [19], the relevant checklists were utilized.
Data synthesis
Meta-analysis was performed using the comprehensive meta-analysis (CMA) software [20] with mean and SD for changes in ICP (in percent) by HTS. A random effect model was utilized for the meta-analysis. 95% confidence intervals (CIs) and 0.05 level of significance for p-value were observed and the result was presented as the forest plot. Also, the publication bias was assessed using the Begg and Mazumdar's correlation test [21] and presented as the funnel plot.
Results
Study inclusion
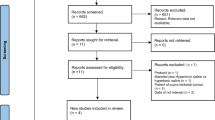
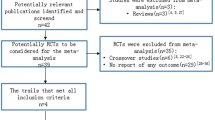
The details of the selection process are presented in the PRISMA flow diagram (Fig. 1). In summary, out of 1321 results of databases searching, 8 studies were included in this systematic review [22,23,24,25,26,27,28,29] and 3 of them were included in the quantitative synthesis. Three of these studies were observational studies and the rest five studies had a clinical trial study design. The publication years were 1998 to 2018. The sample size in these studies was between 6 and 60 and the mean age of the participants varies between 30 and 55. 90-day neurologic status was reported in one study [25], Cottenceau reported a 6-month follow-up, and finally, and Jagannatha et al. only assessed this outcome over 6 days. Table 1 shows a summary of the characteristics and findings of the included studies.
Risk of bias
Table 2 shows the results of the risk of bias assessments using the JBI checklists [18]. Based on our assessments, the most common source of bias in our included RCTs was appropriate reporting blinding methods for both patients and outcome assessors. In one of the cross-sectional studies, dealing with confounding variables was a source of bias. In one quasi-experimental study, there was no control group.
Summary of findings
A similar efficacy for mannitol and HTS in patients with sustained ICP was reported in 3 studies [22, 24, 28]. In two studies, the daily ICP burden was significantly lower in the HTS group compared to Mannitol [23, 25]. In Jagannatha et al.’s study, Mannitol and HTS had a similar effect on ICP over 6 days, but an increase in the daily mean ICP was observed after this span which was significant only in the Mannitol group [27]. Regarding the different doses of HTS, Chris Carter et al. in a study published in 2017 reported the same efficacy for 5% and 23.4% NaCl for a sustained ICP > 20 mm Hg [26]. Finally, in Schatzmann et al.’s study infusions of HTS decreased ICP effectively [29].
Regarding mortality, a similar mortality rate between HTS and Mannitol was reported in 3 studies [23, 25, 27]. Also, the duration of ICU or hospital stays was not significantly different between HTS and Mannitol in 2 studies [23, 27]. Finally, the neurologic outcome did not differ significantly between HTS and Mannitol in 3 studies that reported this outcome [25, 27, 28].
Meta-analysis
A meta-analysis of three studies in which a decrease in ICP was reported in patients receiving HTS was performed. Heterogeneity between studies was not significant (Q-value = 0.187, df = 2, p-value = 0.98, I2 = 0.00%). The results of quantitative synthesis reached a 35.9% (95% CI 15.0–56.9) reduction in ICP in TBI patients receiving HTS (Fig. 2). Figure 3 shows the funnel plot to examine the publication bias which was not significant in included studies (t-value = 0.38, df = 2, p-value = 0.73).
Discussion
This study considers the effectiveness of HTS in the management of elevated ICP secondary to TBI, lowering mortality rates, and improving neurological outcomes. Based on the available evidence, HTS seems to be efficacious in reducing ICP [22,23,24,25,26,27,28,29]. The results of our meta-analysis reported a 35.9% reduction in ICP in TBI patients with HTS therapy. Also, the neurological consequences [25, 27, 28] and mortality rates [23, 25, 27] do not seem to be significantly different between HTS and Mannitol.
From the mechanism point of view, HTS causes plasma expansion by redistribution of fluid from the extravascular space. Also, the immunomodulatory and anti-inflammatory effects of HTS are reported in previous studies [11, 30,31,32]. HTS and mannitol share similar mechanisms for lowering elevated ICP by establishing an osmotic gradient across the blood–brain barrier. Increased brain oxygenation is another mechanism suggested by previous studies [33]. Cottenceau et al. reported no effects of mannitol or HTS in boosting the cerebral metabolism, which was assessed by oxygen, glucose, and lactate levels [28]. Also in Jagannatha et al.’s study, sodium level, osmolality, and renal function parameters were comparable between HTS and Mannitol groups [27]. Higher reflection coefficient, effective maintenance of plasma volume, and dehydration of endothelial cells were also suggested as theoretical advantages of HTS [27, 34, 35].
With the implementation of new guidelines since the 1980s, the management of TBI patients appears to be evolving [36]. Despite the absence of Class 1 evidence, ICP monitoring is suggested as a standard clinical observation in TBI patients [37]. A recent sco** review found the evidence regarding the HTS usage in patients with moderate TBI without ICP monitoring inconclusive [38]. We investigated the role of HTS in controlling ICP as the primary outcome and we found that HTS can significantly reduce the ICP in patients with TBI.
Increased ICP is a common life-threatening condition, which is considered the “silent epidemic” [2]. Studies found average ICP in the first two days is an independent predictor of mortality in patients with severe TBI [39]. In addition, the complications of increased ICP include but are not limited to neurological and visual abnormalities, headaches, and nausea [40]. Therefore, this medical and surgical emergency requires prompt recognition and management [41]. Mannitol as a hyperosmolar therapy with wide usage in TBI [42] is considered the gold standard therapy for increased ICP due to TBI, but experts believe it is due to its historical use and not its superiority over HTS [43]. Recent systematic reviews compared mannitol with HS for treating elevated ICP after TBI and found no superiority for none of them [44, 45]. Diuretic properties of mannitol and hypotension reduce the tendency to Mannitol among clinicians. In addition, poor glycemic control with Mannitol also was reported in studies which may affect the overall result of management [27]; therefore, the latest guidelines recommend HTS over Mannitol [46, 47]. The comparison of HTS and Mannitol in this study found HTS as safe and effective as Mannitol; therefore, the choice between them should be based on circumstances, availability, or the clinical situation [48].
Reduction of ICP can be safely achieved with HTS [49]. Francony et al. observed a prolonged duration of ICP reduction in 120 min [22]. Horn et al. also reported that repeated bolus application of HTS could significantly decrease ICP in patients with therapy-resistant elevation of ICP [50]. This finding was in the same line as Munar et al.’s study which found administration of 7.2% HTS effective in reducing ICP [51]. Appropriate reduction in ICP can lead to the prevention of secondary injury and potentially severe complications.
Mannitol is suggested to have a beneficial effect on mortality [52]. The mortality rates were reported in 3 studies. In Cheng et al.’s study and Vialet et al.’s RCT, mortality was not significantly differed between HTS and Mannitol [23, 25]. A comparable difference in 6 months and in-hospital mortality between mannitol and HTS groups was also reported in Jagannatha’s RCT [27]. In this condition and based on the available evidence it seems Mannitol and HTS have no significant superiority over each other in reducing the mortality rate in patients with TBI; however, there is still a need for additional research in this regard [47, 53].
Improvement of neurological outcomes by HTS was reported previously [32]. In our included studies these outcomes were reported in 3 studies. In Vialet et al.’s RCT, neurologic outcomes based on the number of patients with severe Glasgow scale did not differ significantly between Mannitol and HTS [25]. In Cottenceau et al.’s study, the authors did not detect a significant difference in neurological outcome at 6 months, too [28]. Finally, Jagannatha’s RCT also found similar Glasgow scale scores at ICU and hospital discharge, in Mannitol and HTS groups [27], which was similar to the previously mentioned study. The latest Neurocritical Care Society (NCS) guidelines recommended future studies for a comprehensive conclusion in this regard [47].
Studies assessed the efficacy of prehospital HTS resuscitation on neurological outcomes, too. In a RCT conducted by Cooper et al. in 2004, the authors found almost identical neurological function after 6 months, for conventional resuscitation protocols and HTS, which did not support the routine usage of HTS in the prehospital setting [54].
In addition to hyperosmolar therapy, cerebrospinal fluid drainage and barbiturates are also suggested for the management of patients with TBI. Decompressive craniectomy is also suggested as second-line therapy for elevated ICP. A recent Cochrane review assessed the efficacy of this procedure and found it effective in reducing mortality; nevertheless, the authors found the effects on long-term neurological outcomes controversial [7]. Based on the latest guidelines, decompressive craniectomy is only recommended for late refractory ICP elevation [55].
This study was associated with multiple limitations. The limited number of well-designed RCTs, lack of appropriate reports of serum levels of metabolic parameters, such as sodium and glucose, as well as systemic hemodynamics were the main limitations of this study. Also, different reporting methods prevented a comprehensive meta-analysis. We suggest future well-designed prospective multicenter studies with larger sample sizes, appropriate identification and dealing with possible confounding variables, additional cost–benefit analysis, and a longer duration of follow-up—to translate into a long‐term benefit––for reaching more comprehensive and conclusive results on this topic.
Conclusion
Hyperosmolar therapy with HTS can reduce the ICP significantly, which may lead to the prevention of secondary injury in TBI patients. Based on the available evidence, HTS has relatively similar efficacy to Mannitol, which is considered the gold standard therapy for TBI, in boosting patients’ neurological condition and reducing mortality rates.
Availability of data and materials
All the supporting data and information are available within the manuscript.
References
Faul M, Coronado V. Epidemiology of traumatic brain injury. In: Grafman J, Salazar AM, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier; 2015.
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019;130(4):1080–97.
Dismuke CE, Walker RJ, Egede LE. Utilization and cost of health services in individuals with traumatic brain injury. Glob J Health Sci. 2015;7(6):156–69.
Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018;62(5):535–41.
Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia AK. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. 2018;17(9):689–95.
Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin. 2017;97(5):1015–30.
Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD003983.pub3.
Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–62.
Huang SJ, Chang L, Han YY, Lee YC, Tu YK. Efficacy and safety of hypertonic saline solutions in the treatment of severe head injury. Surg Neurol. 2006;65(6):539–46.
Vedantam A, Gopinath SP. Osmotic therapy in traumatic brain injury. Curr Trauma Rep. 2018;4(2):121–6.
Banks CJ, Furyk JS. Review article: hypertonic saline use in the emergency department. Emerg Med Australas. 2008;20(4):294–305.
Adamides AA, Winter CD, Lewis PM, Cooper DJ, Kossmann T, Rosenfeld JV. Current controversies in the management of patients with severe traumatic brain injury. ANZ J Surg. 2006;76(3):163–74.
Hinson HE, Stein D, Sheth KN. Hypertonic saline and mannitol therapy in critical care neurology. J Intensive Care Med. 2013;28(1):3–11.
Berger-Pelleiter E, Émond M, Lauzier F, Shields J-F, Turgeon AF. Hypertonic saline in severe traumatic brain injury: a systematic review and meta-analysis of randomized controlled trials. CJEM. 2016;18(2):112–20.
Chen H, Song Z, Dennis JA. Hypertonic saline versus other intracranial pressure-lowering agents for people with acute traumatic brain injury. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD010904.pub3.
Aromataris E, Munn Z. 2020 Chapter 1: JBI Systematic Reviews. In: Aromataris E, Munn Z (Eds). JBI Manual for Evidence Synthesis. JB, Adelaide https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-02
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. 2020 Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z (Eds). JBI Manual for Evidence Synthesis. JBI, Adelaide. https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-04.
Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, Tufanaru C, Qureshi R, Mattis P, Mu P. Conducting systematic reviews of association (etiology): the Joanna Briggs institute’s approach. Int J Evid Based Healthc. 2015;13(3):163–9.
Borenstein M. Comprehensive Meta-Analysis Software. In: Egger Matthias, Higgins Julian P.T., Smith George Davey, editors. Systematic Reviews in Health Research. Hoboken: Wiley; 2022.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, Jacquot C, Payen JF. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36(3):795–800.
Cheng F, Xu M, Liu H, Wang W, Wang Z. A retrospective study of intracranial pressure in head-injured patients undergoing decompressive Craniectomy: a comparison of hypertonic saline and mannitol. Front Neurol. 2018. https://doi.org/10.3389/fneur.2018.00631.
Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57(4):727–36.
Vialet R, Albanèse J, Thomachot L, Antonini F, Bourgouin A, Alliez B, Martin C. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31(6):1683–7.
Carter C, Human T. Efficacy, safety, and timing of 5% sodium chloride compared with 23.4% sodium chloride for osmotic therapy. Ann Pharmacother. 2017;51(8):625–9.
Jagannatha AT, Sriganesh K, Devi BI, Rao GSU. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. J Clin Neurosci. 2016;27:68–73.
Cottenceau V, Masson F, Mahamid E, Petit L, Shik V, Sztark F, Zaaroor M, Soustiel JF. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2011;28(10):2003–12.
Schatzmann C, Heissler HE, König K, Klinge-Xhemajli P, Rickels E, Mühling M, Börschel M, Samii M. Treatment of elevated intracranial pressure by infusions of 10% saline in severely head injured patients. Acta Neurochir Suppl. 1998;71:31–3.
Kennedy MT, Higgins BD, Costello JF, Curtin WA, Laffey JG. Hypertonic saline reduces inflammation and enhances the resolution of oleic acid induced acute lung injury. BMC Pulm Med. 2008;8(1):9.
Staudenmayer KL, Maier RV, Jelacic S, Bulger EM. Hypertonic saline modulates innate immunity in a model of systemic inflammation. Shock. 2005. https://doi.org/10.1097/01.shk.0000160523.37106.33.
Tyagi R, Donaldson K, Loftus CM, Jallo J. Hypertonic saline: a clinical review. Neurosurg Rev. 2007;30(4):277–89.
Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009;65(6):1035–41.
Ziai WC, Toung TJK, Bhardwaj A. Hypertonic saline: first-line therapy for cerebral edema? J Neurol Sci. 2007;261(1):157–66.
Himmelseher S. Hypertonic saline solutions for treatment of intracranial hypertension. Curr Opin Anaesthesiol. 2007;20(5):414–26.
Jiang J-Y, Gao G-Y, Feng J-F, Mao Q, Chen L-G, Yang X-F, Liu J-F, Wang Y-H, Qiu B-H, Huang X-J. Traumatic brain injury in China. Lancet Neurol. 2019;18(3):286–95.
Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Anal. 2008. https://doi.org/10.1213/01.ane.0000297296.52006.8e.
Rossong H, Hasen M, Ahmed B, Zeiler FA, Dhaliwal P. Hypertonic saline for moderate traumatic brain injury: a sco** review of impact on neurological deterioration. Neurotrauma Rep. 2020;1(1):253–60.
Badri S, Chen J, Barber J, Temkin NR, Dikmen SS, Chesnut RM, Deem S, Yanez ND, Treggiari MM. Mortality and long-term functional outcome associated with intracranial pressure after traumatic brain injury. Intensive Care Med. 2012;38(11):1800–9.
Haider MN, Leddy JJ, Hinds AL, Aronoff N, Rein D, Poulsen D, Willer BS. Intracranial pressure changes after mild traumatic brain injury: a systematic review. Brain Inj. 2018;32(7):809–15.
Hassan A, Diringer MN. Elevated Intracranial Pressure, Management of. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of the Neurological Sciences. Amsterdam: Elsevier; 2014.
Knapp JM. Hyperosmolar therapy in the treatment of severe head injury in children: mannitol and hypertonic saline. AACN Clin Issues. 2005;16(2):199–211.
Boone MD, Oren-Grinberg A, Robinson TM, Chen CC, Kasper EM. Mannitol or hypertonic saline in the setting of traumatic brain injury: what have we learned? Surg Neurol Int. 2015;6:177.
Gu J, Huang H, Huang Y, Sun H, Xu H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: a meta-analysis of randomized controlled trials. Neurosurg Rev. 2019;42(2):499–509.
Rickard AC, Smith JE, Newell P, Bailey A, Kehoe A, Mann C. Salt or sugar for your injured brain? A meta-analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury. Emerg Med J. 2014;31(8):679–83.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15.
Cook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, Samuel S, Tokumaru S, Venkatasubramanian C, Zacko C, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care. 2020;32(3):647–66.
Quintard H, Meyfroidt G, Citerio G. hyperosmolar agents for TBI: all are equal, but some are more equal than others? Neurocrit Care. 2020;33(2):613–4.
Strandvik GF. Hypertonic saline in critical care: a review of the literature and guidelines for use in hypotensive states and raised intracranial pressure*. Anaesthesia. 2009;64(9):990–1003.
Horn P, Münch E, Vajkoczy P, Herrmann P, Quintel M, Schilling L, Schmiedek P, Schürer L. Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurol Res. 1999;21(8):758–64.
Munar F, Ferrer AM, de Nadal M, Poca MA, Pedraza S, Sahuquillo J, Garnacho A. Cerebral hemodynamic effects of 7.2% hypertonic saline in patients with head injury and raised intracranial pressure. J Neurotrauma. 2000;17(1):41–51.
Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD001049.pub5.
Cook AM, Shutter L. Response to Drs Quintard, et al. Neurocritical Care. 2020;33(2):615–6.
Cooper DJ, Myles PS, McDermott FT, Murray LJ, Laidlaw J, Cooper G, Tremayne AB, Bernard SS, Ponsford J. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291(11):1350–7.
Hawryluk GWJ, Rubiano AM, Totten AM, O’Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87(3):427–34.
Acknowledgements
The research protocol was approved and supported by the Emergency and Trauma Care Research Center, Tabriz University of Medical Sciences (Grant number: 64363). Also, the authors thank the clinical research development unit of Imam Reza hospital, Tabriz University of Medical Sciences for their kind supports.
Author contributions
NG contributed to investigation; resources; writing—original draft; funding acquisition; and project administration; MG was involved in methodology; formal analysis; and visualization; FT contributed to writing—original draft; investigation; and resources; SD was involved in investigation; writing—review & editing; supervision; validation; funding acquisition; and project administration; HS was involved in writing—review & editing; supervision; conceptualization; and validation. All the authors read and approved the final manuscript.
Funding
This study was supported by the Deputy for Research of Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
NG: Investigation; Resources; Writing - Original Draft; Funding acquisition; Project administration; MG: Methodology; Formal analysis; Visualization; AN, FT: Writing - Original Draft; Investigation; Resources; SD: Investigation; Writing - Review & Editing; Supervision; Validation; Funding acquisition; Project administration; HS: Writing - Review & Editing; Supervision; Conceptualization; Validation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research protocol was reviewed and approved by the ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.1227).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Details of search strategies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gharizadeh, N., Ghojazadeh, M., Naseri, A. et al. Hypertonic saline for traumatic brain injury: a systematic review and meta-analysis. Eur J Med Res 27, 254 (2022). https://doi.org/10.1186/s40001-022-00897-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00897-4