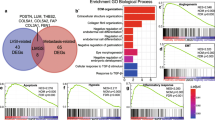
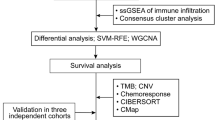
Abstract
Background
Epithelial ovarian cancer is the leading cause of death from gynecologic cancer, in which serous ovarian carcinoma (SOC) is the most common histological subtype. Although PARP inhibitors (PARPi) and antiangiogenics have been accepted as maintenance treatment in SOC, response to immunotherapy of SOC patients is limited.
Methods
The source of transcriptomic data of SOC was from the Cancer Genome Atlas database and Gene Expression Omnibus. The abundance scores of mesenchymal stem cells (MSC scores) were estimated for each sample by xCell. Weighted correlation network analysis is correlated the significant genes with MSC scores. Based on prognostic risk model construction with Cox regression analysis, patients with SOC were divided into low- and high-risk groups. And distribution of immune cells, immunosuppressors and pro-angiogenic factors in different risk groups was achieved by single-sample gene set enrichment analysis. The risk model of MSC scores was further validated in datasets of immune checkpoint blockade and antiangiogenic therapy. In the experiment, the mRNA expression of prognostic genes related to MSC scores was detected by real-time polymerase chain reaction, while the protein level was evaluated by immunohistochemistry.
Results
Three prognostic genes (PER1, AKAP12 and MMP17) were the constituents of risk model. Patients classified as high-risk exhibited worse prognosis, presented with an immunosuppressive phenotype, and demonstrated high micro-vessel density. Additionally, these patients were insensitive to immunotherapy and would achieve a longer overall survival with antiangiogenesis treatment. The validation experiments showed that the mRNA of PER1, AKAP12, and MMP17 was highly expressed in normal ovarian epithelial cells compared to SOC cell lines and there was a positive correlation between protein levels of PER1, AKAP12 and MMP17 and metastasis in human ovarian serous tumors.
Conclusion
This prognostic model established on MSC scores can predict prognosis of patients and provide the guidance for patients receiving immunotherapy and molecular targeted therapy. Because the number of prognostic genes was fewer than other signatures of SOC, it will be easily accessible on clinic.
Similar content being viewed by others
Background
Ovarian cancer (OC) is one of the most dangerous gynecologic malignancies. In 2020, the morbidity of OC was estimated to be 3.4% worldwide for women, and ranked eighth among female malignant tumors and third among gynecological malignancies. Mortality from OC accounts for 4.7% of all female malignant tumors around the whole world and is ranked second among female genital tumors [1]. Serous ovarian cancer (SOC) is the most common type of gynecological malignancy [2]. The National Comprehensive Cancer Network (NCCN) has recommended poly ADP-ribose polymerase inhibitors (PARPi) and angiogenesis inhibitors as treatment for patients with SOC. Although PARPi and antiangiogenics have been shown to prolong progression-free survival (PFS), they do not reflect an obvious improvement in overall survival (OS) of patients with SOC [3, 4].
The tumor microenvironment (TME) denotes the niche where tumor cells interact with the surrounding stroma, including various immune cells, stroma cells, lymph-vascular space, and the extracellular matrix (ECM) [5]. Mesenchymal stem cells (MSCs) are a key component of stromal cells and can mediate the immune response by inhibiting the activity of T lymphocytes, interfering with the proliferation and differentiation of B lymphocytes, and inducing macrophage phenotypic switching [6]. In addition to immune regulation, MSCs can promote angiogenesis by releasing soluble factors and can be a source of carcinoma-associated fibroblasts (CAF). CAFs, in turn, can directly release angiogenesis-related factors and indirectly modulate pathophysiological processes, including ECM stiffness, elasticity, and interstitial fluid pressure [7, 8]. In OC, MSCs regulate cancer cell proliferation, metastasis, phenotype, and response to chemotherapy by binding directly to target cells, secreting soluble factors, or discharging exosomes from MSCs [9,10,11]. Cancer-associated MSCs (CA-MSCs) can be isolated and identified in tumor tissue, and exhibit a unique gene expression profile from MSCs compared to that of healthy individuals [12]. Patients with the CA-MSC phenotype have a significantly worse PFS than those with the normal MSC phenotype [10]. It is reported that CA-MSCs of an immune 'hot' mouse OC drived CD8 + T cell tumor immune evasion of CD8 + T cells from tumors and these mouse exhibited a poor response to anti-programmed death ligand 1 (PD-L1) immune checkpoint blockade therapy (ICB) through the secretion of multiple chemokines, such as CCL2, CX3CL1, and TGF-β1 [13].
Cobalt chloride (CoCl2)-induced polyploid giant cancer cells (PGCC) exhibit the same characteristics as cancer stem cells (CSC) and express markers related to CSC (CD133 and CD44). Daughter cells produced by PGCC undergo an epithelial–mesenchymal transition (EMT) and gain a mesenchymal phenotype and are thus endowed with strong abilities for migration and invasion [43], which may be related to a poor prognosis of the mesenchymal phenotype of SOC. For different MSC phenotypes, high-risk scores indicated the presence of abundant infiltration of immunocytes with inhibitory activity, such as immature dendritic cells and regulatory T cells (Fig. 4C). Except for tumor-associated immunosuppressive cells, immunity inhibition factors were also differentially expressed (Fig. 4D; Additional file 16: Fig. S4A). MSCs have been reported to secrete immunomodulatory factors that influence other immune or stromal cells, such as transforming growth factor-beta (TGF-β1) on macrophages, vascular endothelial growth factor receptor 2 (KDR) in endothelial cells, and galectin-9 (LGALS9) in T cells [51]. TAMs are a heterogeneous cell population and broadly classified into pro-inflammatory M1 and anti-inflammatory M2 macrophages. In OC, M2 macrophages comprise 39% of the immune cells and are associated with adverse clinical outcomes [52]. Single cell spatial analysis discloses intimate interactions of exhausted CD8+ T cells and PD-L1+ macrophages that are considered mechanistic determinants of response to niraparib and pembrolizumab treatment, which are PARP and immune checkpoint inhibitors, respectively [53]. Among stromal cells, CAFs originate from MSCs or by transdifferentiation of other cells. With the expression of specific molecules and receptors, CAFs promote angiogenesis, metastasis, and infiltration of immunosuppressive cells, thus fueling tumor growth and progression [54]. SOC stromal fibroblasts exhibit intrinsic resistance to PARPi and increased further after PARPi administration [55]. Except for the PARPi response, patients with high CAF infiltration exhibit chemoresistance and contribute to the insensitivity to immunotherapy [54, 56, 57]. Similarly, the increased dispersion of MSCs in SOCs tends to shorten survival and attenuates the response to immunotherapy of patients in our study.
In terms of the clinical relevance, the expression of MSC markers and the existence of soluble factors derived from MSCs are negatively correlated with the prognosis of patients. CD105 + MSCs were associated with a reduced OS of patients with brain neoplasm, lung cancer, and gastric cancer [58,59,60]. MMP9 and IL-6 are secreted proteins of MSCs. High expression of MMP9 has been associated with low survival rates in lung adenocarcinoma [61]. Patients with high IL-6 levels have significantly a poorer survival rate than those with low IL-6 levels [62]. The exosomal microRNAs released by MSCs were positively related to survival time in colorectal, myeloid leukemia, nasopharyngeal carcinoma, and glioma [63,64,65,66].
Essentially, MSCs exert immunomodulatory effects on both innate and adaptive cells through cell-to-cell contact and paracrine activity, including T cells, natural killer (NK) cells, and DCs. Induction of regulatory T cells (Tregs) is a main mechanism of immunosuppression by MSCs. MSCs can convert conventional T cells (T convs) to Forkhead box P3 (Foxp3) expressing Tregs [67]. Foxp3 is a transcription factor that inimitably defines Tregs and is a requirement for Tregs differentiation [68]. The immature dendritic cells are a subset of dendritic cells that selectively promote the proliferation of Tregs, and both take part in immunosuppressive activity [69]. Moreover, mature DCs co-cultured with MSCs skew to immature status and show a reduced stimulatory activity on T cells [70]. Therefore, in our study, patients with the mesenchymal phenotype tended to have an immunosuppressive state characterized by a richness of Tregs and immature DCs, and responded poorly to anti-PD-1 therapy. However, there are other immunoeffector cells that assembled in the high-risk score group. NK cells are innate cytotoxic lymphocytes and MSCs modulate their inhibitory effects on cell proliferation, altered cytotoxicity and cytokine production, and induction of apoptosis by MSC secreted cytokines such as prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), TGF-β1, IL-6, and nitric oxide (NO) [71]. In our study, NK cells were enriched in the high MSC risk score group. This change largely resulted from the fact that there was heterogeneity among NK cells, and CD56bright NK cells and CD56dim NK cells are two main subsets of circulating human NK cells. CD56bright NK cells are more immature and are more enriched in the tumor, and exhibit more limited cytotoxicity responses compared to CD56dim NK cells [72], which is likely to occupy the majority of the mesenchymal phenotype of OC. In addition, Wan et al. revealed that the unique bispecific anti-programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) antibody induced NK cells to transition from inert to more active and cytotoxic phenotypes, implicating NK cells as the key missing component of the current ICB-induced immune response in SOC [73].
With the exception of the prognostic response to ICB, our MSC score system is able to provide guidance for anti-angiogenesis therapy. As for patients with high-risk scores, standard chemotherapy plus angiogenic inhibitor is superior to chemotherapy alone. Stefani et al. found that low-dose irradiated MSCs showed antiangiogenic properties and infiltrated predominantly the perivascular niche, leading to rejection of established tumors [74]. MiR-16, a microRNA targeting VEGF, was enriched in MSC-derived exosomes and partially resulted in an antiangiogenic effect in breast cancer cells [75]. The MSC score system was a prospective marker for the administration of PARPi. The higher the risk scores, the lower the number of defects in homologous recombination, for which repair deficiency is closely associated with sensitivity to PARPi therapy in epithelial OC [76, 77]. Therefore, patients with a mesenchymal phenotype may not be suitable for treatment with PARPi.
In our validation experiment, three genes related to poor outcomes were prone to accumulate in the healthy ovary and in epithelial cells. However, the PER1 content is distinct between HEY and SKOv3 cells, which may be explained by the role of TP53. TP53 in HEY cells is wild-type and in SKOv3 cells is deleted. PER1 knockdown influences pancreatic cancer cell lines with mutated TP53, but does not alter cells containing wild-type TP53 [78], the reason for this finding is that p53 represses PER1 transcription [79]. MSCs can act directly on metastasis of tumor through production of pro-metastatic cytokines or regulation of epithelial-mesenchymal transition [80, 81]. Not only in SOC, the elevated proteins of PER1, AKAP12 and MMP17 are also associated with the migration and invasion in triple-negative breast cancer, melanoma and colon cancer [82,83,84].
Because of its multipotency, low immunogenicity, easy accessibility and ethical advantage compared to pluripotent stem cells or embryonic stem cells, MSCs are desirable candidates for in degenerative and inflammatory diseases, auto-immune diseases, such as joint injury, atopic dermatitis, and multiple sclerosis [85]. Infrapatellar fat pad-derived mesenchymal stem cells, proximal to the knee joint and similar to adipose cells, own proliferation and differentiation potential independent of age and promote hyaline-like cartilage formation without integration into the surrounding cartilage [86]. Currently, there are several phase I/II and III clinical trials involving immunomodulatory MSCs aimed at treating graft-versus-host disease and tumors. In combination with ganciclovir, genetically-modified autologous MSC were found to be safe and tolerable in patients with advanced gastrointestinal adenocarcinoma [87]. A similar trial confirmed that allogeneic MSC infusions showed safety and feasibility in patients with prostate cancer [88]. Another trial in which endovascular superselective intraarterial (ESIA) MSC infusions loaded with an oncolytic adenovirus Delta-24 (MSC-D24) were used to treat glioblastoma is currently underway [89]. The direction of treatment for MSCs mainly includes the delivery of various anticancer biological agents or suicide genes using an extracellular vesicle derived from MSCs [90, 91]. However, we have to face the possibility about the latent pro-metastasis functions and the promotion of immune evasion if anticancer agents or suicide genes in MSCs cannot function. In addition, MSCs combined with drug nanoparticles were used to induce the death of cancer cells. The conjugation forms between MSCs and nanoparticles include MSCs loading nanoparticles, nanoparticles attached to MSCs surface, nanoparticles coated with MSCs membrane, and vectors of anti-tumor genes in MSCs [92]. Because of tumor tropism of MSCs, the conjugation between MSCs and nanoparticles solved the problem of low target specificity and minimized side effects of conventional medicine. However, the toxicity of nanoparticles and the uncertainty of pharmacokinetics are still existent, including accumulation in organs followed by inflammation or binding with blood constituent followed by coagulation [93]. If these disadvantages about the safety of MSCs, nanoparticles or drugs can be solved, MSC may gradually be applied in clinical practice.
Conclusions
Using a comprehensive transcriptomic analysis of genes characterizing MSCs, this study constructed an MSC-related prognostic model that could separate patients into two groups. The high-risk group was associated with a worse prognosis, a different immunosuppressive phenotype, and a weak response to anti-PD-1 treatment. There was also instructive significance of this sorting system for anti-angiogenesis therapy.
Availability of data and materials
The datasets used during the current study are available in the TCGA, GEO, UCAC Xena and TISIDB website. All data analysed during this study are included in this published article.
Abbreviations
- SOC:
-
Serous ovarian carcinoma
- PARPi:
-
Poly ADP-ribose polymerase inhibitor
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- TME:
-
Tumor microenvironment
- MSC:
-
Mesenchymal stem cell
- CAF:
-
Carcinoma-associated fibroblast
- CA-MSC:
-
Cancer-associated mesenchymal stromal cell
- PD-L1:
-
Programmed death ligand 1
- PD-1:
-
Programmed death 1
- ICB:
-
Immune checkpoint blockade
- HRD:
-
Homologous defect recombination
- FPKM:
-
Fragments per kilobase million
- TCGA:
-
The Cancer Genome Atlas
- GEO:
-
Gene Expression Omnibus
- GTEx:
-
Genotype-tissue expression
- TPM:
-
Transcripts per million
- MSigDB:
-
Molecular signatures database
- ssGSEA:
-
Single sample gene set enrichment analysis
- DEG:
-
Differentially expressed gene
- FDR:
-
False discover rate
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- WGCNA:
-
Weighted correlation network analysis
- MMP17:
-
Matrix metallopeptidase 17
- AKAP12:
-
A-kinase anchoring protein 12
- PER1:
-
Period circadian regulator 1
- ITH:
-
Intratumor heterogeneity
- CNV:
-
Copy number variation
- TMB:
-
Tumor mutation burden
- DCA:
-
Decision curve analysis
- PGCC:
-
Polyploid giant cancer cell
- IHC:
-
Immunohistochemistry
- BP:
-
Biological process
- CC:
-
Cellular component
- MF:
-
Molecular function
- DC:
-
Dendritic cell
- TAM:
-
Tumor associated macrophage
- MDSC:
-
Myeloid-derived suppressor cell
- NK:
-
Natural killer
- Tregs:
-
Regulatory T cells
- PGE2:
-
Prostaglandin E2
- IDO:
-
Indoleamine 2,3-dioxygenase
- NO:
-
Nitric oxide
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Clarke CL, Kushi LH, Chubak J, Pawloski PA, Bulkley JE, Epstein MM, Burnett-Hartman AN, Powell B, Pearce CL, Spencer Feigelson H. Predictors of long-term survival among high-grade serous ovarian cancer patients. Cancer Epidemiol Biomarkers Prevent. 2019;28:996–9.
Tattersall A, Ryan N, Wiggans AJ, Rogozińska E, Morrison J. Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst Rev. 2022;2:CD007929.
An D, Banerjee S, Lee J-M. Recent advancements of antiangiogenic combination therapies in ovarian cancer. Cancer Treat Rev. 2021;98: 102224.
Xu L, Zou C, Zhang S, Chu TSM, Zhang Y, Chen W, Zhao C, Yang L, Xu Z, Dong S, Yu H, Li B, Guan X, Hou Y, Kong F-M. Resha** the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol. 2022;15:87.
Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal stem/stromal cells and their paracrine activity-immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics. 2022;14:381.
Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195.
Meng Q, Luo X, Chen J, Wang D, Chen E, Zhang W, Zhang G, Zhou W, Xu J, Song Z. Unmasking carcinoma-associated fibroblasts: key transformation player within the tumor microenvironment. Biochim Biophys Acta Rev Cancer. 2020;1874: 188443.
Wang X, Jiang L, Liu Q. miR-18a-5p derived from mesenchymal stem cells-extracellular vesicles inhibits ovarian cancer cell proliferation, migration, invasion, and chemotherapy resistance. J Transl Med. 2022;20:258.
Fan H, Atiya HI, Wang Y, Pisanic TR, Wang TH, Shih IM, Foy KK, Frisbie L, Buckanovich RJ, Chomiak AA, Tiedemann RL, Rothbart SB, Chandler C, Shen H, Coffman LG. Epigenomic reprogramming toward mesenchymal–epithelial transition in ovarian-cancer-associated mesenchymal stem cells drives metastasis. Cell Rep. 2020;33: 108473.
Raghavan S, Snyder CS, Wang A, McLean K, Zamarin D, Buckanovich RJ, Mehta G. Carcinoma-associated mesenchymal stem cells promote chemoresistance in ovarian cancer stem cells via PDGF signaling. Cancers. 2020;12:2063.
McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, Buckanovich RJ. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–19.
Cascio S, Chandler C, Zhang L, Sinno S, Gao B, Onkar S, Bruno TC, Vignali DAA, Mahdi H, Osmanbeyoglu HU, Vlad AM, Coffman LG, Buckanovich RJ. Cancer-associated MSC drive tumor immune exclusion and resistance to immunotherapy, which can be overcome by Hedgehog inhibition. Sci Adv. 2021;7:eabi5790.
Zhang S, Mercado-Uribe I, **ng Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–28.
Li Z, Zheng M, Zhang H, Yang X, Fan L, Fu F, Fu J, Niu R, Yan M, Zhang S. Arsenic trioxide promotes tumor progression by inducing the formation of PGCCs and embryonic hemoglobin in colon cancer cells. Front Oncol. 2021;11: 720814.
Liu G, Wang Y, Fei F, Wang X, Li C, Liu K, Du J, Cao Y, Zhang S. Clinical characteristics and preliminary morphological observation of 47 cases of primary anorectal malignant melanomas. Melanoma Res. 2018;28:592–9.
Zhang S, Mercado-Uribe I, Sood A, Bast RC, Liu J. Coevolution of neoplastic epithelial cells and multilineage stroma via polyploid giant cells during immortalization and transformation of Mullerian epithelial cells. Genes Cancer. 2016;7:60–72.
Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–15.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43: e47.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Davidson BA, Rubatt JM, Corcoran DL, Teoh DK, Bernardini MQ, Grace LA, Soper WJ, Berchuck A, Siamakpour-Reihani S, Chen W, Owzar K, Murphy SK, Secord AA. Differential angiogenic gene expression in TP53 wild-type and mutant ovarian cancer cell lines. Front Oncol. 2014;4:163.
Siamakpour-Reihani S, Owzar K, Jiang C, Turner T, Deng Y, Bean SM, Horton JK, Berchuck A, Marks JR, Dewhirst MW, Alvarez Secord A. Prognostic significance of differential expression of angiogenic genes in women with high-grade serous ovarian carcinoma. Gynecol Oncol. 2015;139:23–9.
Bentink S, Haibe-Kains B, Risch T, Fan JB, Hirsch MS, Holton K, Rubio R, April C, Chen J, Wickham-Garcia E, Liu J, Culhane A, Drapkin R, Quackenbush J, Matulonis UA. Angiogenic mRNA and microRNA gene expression signature predicts a novel subtype of serous ovarian cancer. PLoS ONE. 2012;7: e30269.
Mendiola M, Barriuso J, Redondo A, Marino-Enriquez A, Madero R, Espinosa E, Vara JA, Sanchez-Navarro I, Hernandez-Cortes G, Zamora P, Perez-Fernandez E, Miguel-Martin M, Suarez A, Palacios J, Gonzalez-Baron M, Hardisson D. Angiogenesis-related gene expression profile with independent prognostic value in advanced ovarian carcinoma. PLoS ONE. 2008;3: e4051.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, Frohling S, Chan EM, Sos ML, Michel K, Mermel C, Silver SJ, Weir BA, Reiling JH, Sheng Q, Gupta PB, Wadlow RC, Le H, Hoersch S, Wittner BS, Ramaswamy S, Livingston DM, Sabatini DM, Meyerson M, Thomas RK, Lander ES, Mesirov JP, Root DE, Gilliland DG, Jacks T, Hahn WC. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–12.
Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18:220.
Chen Y, Lun AT, Smyth GK. From reads to genes to pathways: differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Res. 2016;5:1438.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, OuYang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS, N. Cancer Genome Atlas Research, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I. The immune landscape of cancer. Immunity. 2018;48:812–83014.
Mounir M, Lucchetta M, Silva TC, Olsen C, Bontempi G, Chen X, Noushmehr H, Colaprico A, Papaleo E. New functionalities in the TCGAbiolinks package for the study and integration of cancer data from GDC and GTEx. PLoS Comput Biol. 2019;15: e1006701.
Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–56.
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, Chan NW, Zhang J. TISIDB: an integrated repository portal for tumor–immune system interactions. Bioinformatics. 2019;35:4200–2.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–74.
Jiang R, Su G, Chen X, Chen S, Li Q, **e B, Zhao Y. Esculetin inhibits endometrial cancer proliferation and promotes apoptosis via hnRNPA1 to downregulate BCLXL and XIAP. Cancer Lett. 2021;521:308–21.
Li YY, Cen H, Gong BN, Mai S, Wang QL, Mou S, Li Y. TCR-induced tyrosine phosphorylation at Tyr270 of SUMO protease SENP1 by Lck modulates SENP1 enzyme activity and specificity. Front Cell Dev Biol. 2021;9: 789348.
Zhang T, Wang C, Wang K, Liang Y, Liu T, Feng L, Yang X. RacGAP1 promotes the malignant progression of cervical cancer by regulating AP-1 via miR-192 and p-JNK. Cell Death Dis. 2022;13:604.
Zhang S, Guo K, Liang Y, Wang K, Liu S, Yang X. ADGRG1 is a predictor of chemoresistance and poor survival in cervical squamous carcinoma. Front Oncol. 2021;11: 671895.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9.
Wang S, **ong Y, Zhao L, Gu K, Li Y, Zhao F, Li J, Wang M, Wang H, Tao Z, Wu T, Zheng Y, Li X, Liu XS. UCSCXenaShiny: an R/CRAN package for interactive analysis of UCSC Xena Data. Bioinformatics. 2021;38:527–9.
Qiu L, Wang J, Chen M, Chen F, Tu W. Exosomal microRNA146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2mediated PI3K/Akt axis. Int J Mol Med. 2020;46:609–20.
Hassan AA, Artemenko M, Tang MKS, Shi Z, Chen LY, Lai HC, Yang Z, Shum HC, Wong AST. Ascitic fluid shear stress in concert with hepatocyte growth factor drive stemness and chemoresistance of ovarian cancer cells via the c-Met-PI3K/Akt-miR-199a-3p signaling pathway. Cell Death Dis. 2022;13:537.
Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225–37.
Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu J, Chen J, Yang Y, **e J. MSC-secreted TGF-beta regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10:345.
Ge Q, Zhang H, Hou J, Wan L, Cheng W, Wang X, Dong D, Chen C, **a J, Guo J, Chen X, Wu X. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol Med Rep. 2018;17:1667–75.
Fan J, Tang X, Wang Q, Zhang Z, Wu S, Li W, Liu S, Yao G, Chen H, Sun L. Mesenchymal stem cells alleviate experimental autoimmune cholangitis through immunosuppression and cytoprotective function mediated by galectin-9. Stem Cell Res Ther. 2018;9:237.
Upadhaya S, Neftelino ST, Hodge JP, Oliva C, Campbell JR, Yu JX. Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov. 2021;20:168–9.
Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu WJ, Gajewski TF, Slingluff CL Jr, Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom JW, Schneck JP, Horak CE, Weinhold N, Chan TA. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171:934-949 e16.
Zhang S, Cheng C, Lin Z, **ao L, Su X, Zheng L, Mu Y, Liao M, Ouyang R, Li W, Ma J, Cai J, Liu L, Wang D, Zeng F, Liu J. The global burden and associated factors of ovarian cancer in 1990–2019: findings from the Global Burden of Disease Study 2019. BMC Public Health. 2022;22:1455.
Jiang Y, Wang C, Zhou S. Targeting tumor microenvironment in ovarian cancer: premise and promise. Biochim Biophys Acta Rev Cancer. 2020;1873: 188361.
Hornburg M, Desbois M, Lu S, Guan Y, Lo AA, Kaufman S, Elrod A, Lotstein A, DesRochers TM, Munoz-Rodriguez JL, Wang X, Giltnane J, Mayba O, Turley SJ, Bourgon R, Daemen A, Wang Y. Single-cell dissection of cellular components and interactions sha** the tumor immune phenotypes in ovarian cancer. Cancer Cell. 2021;39:928-944e6.
Schweer D, McAtee A, Neupane K, Richards C, Ueland F, Kolesar J. Tumor-associated macrophages and ovarian cancer: implications for therapy. Cancers (Basel). 2022;14:2220.
Farkkila A, Gulhan DC, Casado J, Jacobson CA, Nguyen H, Kochupurakkal B, Maliga Z, Yapp C, Chen YA, Schapiro D, Zhou Y, Graham JR, Dezube BJ, Munster P, Santagata S, Garcia E, Rodig S, Lako A, Chowdhury D, Shapiro GI, Matulonis UA, Park PJ, Hautaniemi S, Sorger PK, Swisher EM, D’Andrea AD, Konstantinopoulos PA. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun. 2020;11:1459.
Ding H, Zhang J, Zhang F, Xu Y, Yu Y, Liang W, Li Q. Role of Cancer-Associated fibroblast in the pathogenesis of ovarian Cancer: focus on the latest therapeutic approaches. Int Immunopharmacol. 2022;110: 109052.
Li X, Fang T, Xu S, ** P, Zhou D, Wang Z, Li H, Yang Z, Chen G, Zheng X, **a Y, Wei X, Zhang Z, Yang X, Wang Y, Gao Q. PARP inhibitors promote stromal fibroblast activation by enhancing CCL5 autocrine signaling in ovarian cancer. NPJ Precis Oncol. 2021;5:49.
Wessolly M, Mairinger E, Borchert S, Bankfalvi A, Mach P, Schmid KW, Kimmig R, Buderath P, Mairinger FD. CAF-associated paracrine signaling worsens outcome and potentially contributes to chemoresistance in epithelial ovarian cancer. Front Oncol. 2022;12: 798680.
Zou R, Jiang Q, ** T, Chen M, Yao L, Ding H. Pan-cancer analyses and molecular subtypes based on the cancer-associated fibroblast landscape and tumor microenvironment infiltration characterization reveal clinical outcome and immunotherapy response in epithelial ovarian cancer. Front Immunol. 2022;13: 956224.
Sentek H, Klein D. Lung-resident mesenchymal stem cell fates within lung cancer. Cancers (Basel). 2021;13:4637.
Numakura S, Uozaki H, Kikuchi Y, Watabe S, Togashi A, Watanabe M. Mesenchymal stem cell marker expression in gastric cancer stroma. Anticancer Res. 2019;39:387–93.
Shahar T, Rozovski U, Hess KR, Hossain A, Gumin J, Gao F, Fuller GN, Goodman L, Sulman EP, Lang FF. Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro Oncol. 2017;19:660–8.
Gu JJ, Hoj J, Rouse C, Pendergast AM. Mesenchymal stem cells promote metastasis through activation of an ABL-MMP9 signaling axis in lung cancer cells. PLoS ONE. 2020;15: e0241423.
Castro-Oropeza R, Vazquez-Santillan K, Diaz-Gastelum C, Melendez-Zajgla J, Zampedri C, Ferat-Osorio E, Rodriguez-Gonzalez A, Arriaga-Pizano L, Maldonado V. Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer. Sci Rep. 2020;10:14205.
Qu M, Li J, Hong Z, Jia F, He Y, Yuan L. The role of human umbilical cord mesenchymal stem cells-derived exosomal microRNA-431-5p in survival and prognosis of colorectal cancer patients. Mutagenesis. 2022;37:164.
Jiang D, Wu X, Sun X, Tan W, Dai X, **e Y, Du A, Zhao Q. Bone mesenchymal stem cell-derived exosomal microRNA-7-5p inhibits progression of acute myeloid leukemia by targeting OSBPL11. J Nanobiotechnol. 2022;20:29.
Wan F, Zhang H, Hu J, Chen L, Geng S, Kong L, Lu JJ. Mesenchymal stem cells inhibits migration and vasculogenic mimicry in nasopharyngeal carcinoma via exosomal MiR-125a. Front Oncol. 2022;12: 781979.
Dai X, Wang Y, Dong X, Sheng M, Wang H, Shi J, Sheng Y, Liu L, Jiang Q, Chen Y, Wu B, Yang X, Cheng H, Kang C, Dong J. Downregulation of miRNA-146a-5p promotes malignant transformation of mesenchymal stromal/stem cells by glioma stem-like cells. Aging (Albany NY). 2020;12:9151–72.
Razazian M, Khosravi M, Bahiraii S, Uzan G, Shamdani S, Naserian S. Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells. World J Stem Cells. 2021;13:971–84.
Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development. Annu Rev Immunol. 2020;38:421–53.
Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29.
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6.
Abbasi B, Shamsasenjan K, Ahmadi M, Beheshti SA, Saleh M. Mesenchymal stem cells and natural killer cells interaction mechanisms and potential clinical applications. Stem Cell Res Ther. 2022;13:97.
Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov. 2022;21:559–77.
Wan C, Keany MP, Dong H, Al-Alem LF, Pandya UM, Lazo S, Boehnke K, Lynch KN, Xu R, Zarrella DT, Gu S, Cejas P, Lim K, Long HW, Elias KM, Horowitz NS, Feltmate CM, Muto MG, Worley MJ Jr, Berkowitz RS, Matulonis UA, Nucci MR, Crum CP, Rueda BR, Brown M, Liu XS, Hill SJ. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 2021;81:158–73.
Stefani FR, Eberstal S, Vergani S, Kristiansen TA, Bengzon J. Low-dose irradiated mesenchymal stromal cells break tumor defensive properties in vivo. Int J Cancer. 2018;143:2200–12.
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE. 2013;8: e84256.
Mukhopadhyay A, Plummer ER, Elattar A, Soohoo S, Uzir B, Quinn JE, McCluggage WG, Maxwell P, Aneke H, Curtin NJ, Edmondson RJ. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675–82.
Hardesty MM, Krivak TC, Wright GS, Hamilton E, Fleming EL, Belotte J, Keeton EK, Wang P, Gupta D, Clements A, Gray HJ, Konecny GE, Moore RG, Richardson DL. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol. 2022;166:219–29.
Sato F, Nagata C, Liu Y, Suzuki T, Kondo J, Morohashi S, Imaizumi T, Kato Y, Kijima H. PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem. 2009;146:833–8.
Bellet MM, Stincardini C, Costantini C, Gargaro M, Pieroni S, Castelli M, Piobbico D, Sassone-Corsi P, Della-Fazia MA, Romani L, Servillo G. The circadian protein PER1 modulates the cellular response to anticancer treatments. Int J Mol Sci. 2021;22:2974.
Lin WH, Chang YW, Hong MX, Hsu TC, Lee KC, Lin C, Lee JL. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT-MET switch and cancer metastasis. Oncogene. 2021;40:791–805.
Mohr A, Chu T, Clarkson CT, Brooke GN, Teif VB, Zwacka RM. Fas-threshold signalling in MSCs promotes pancreatic cancer progression and metastasis. Cancer Lett. 2021;519:63–77.
Finger EC, Castellini L, Rankin EB, Vilalta M, Krieg AJ, Jiang D, Banh A, Zundel W, Powell MB, Giaccia AJ. Hypoxic induction of AKAP12 variant 2 shifts PKA-mediated protein phosphorylation to enhance migration and metastasis of melanoma cells. Proc Natl Acad Sci USA. 2015;112:4441–6.
**e F, Wang L, Liu Y, Liu Z, Zhang Z, Pei J, Wu Z, Zhai M, Cao Y. ASMT regulates tumor metastasis through the circadian clock system in triple-negative breast cancer. Front Oncol. 2020;10: 537247.
Yu J, He Z, He X, Luo Z, Lian L, Wu B, Lan P, Chen H. Comprehensive analysis of the expression and prognosis for MMPs in human colorectal cancer. Front Oncol. 2021;11: 771099.
Liu Y, Graves DT, Wang S. Development and clinical application of human mesenchymal stem cell drugs. Sci Bull (Bei**g). 2023;68:860–3.
Vahedi P, Moghaddamshahabi R, Webster TJ, CalikogluKoyuncu AC, Ahmadian E, Khan WS, JimaleMohamed A, Eftekhari A. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: a review. Int J Mol Sci. 2021;22:9215.
von Einem JC, Guenther C, Volk HD, Grutz G, Hirsch D, Salat C, Stoetzer O, Nelson PJ, Michl M, Modest DP, Holch JW, Angele M, Bruns C, Niess H, Heinemann V. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: results from the phase 1/2 TREAT-ME-1 trial. Int J Cancer. 2019;145:1538–46.
Schweizer MT, Wang H, Bivalacqua TJ, Partin AW, Lim SJ, Chapman C, Abdallah R, Levy O, Bhowmick NA, Karp JM, De Marzo A, Isaacs JT, Brennen WN, Denmeade SR. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Transl Med. 2019;8:441–9.
Chen SR, Chen MM, Ene C, Lang FF, Kan P. Perfusion-guided endovascular super-selective intra-arterial infusion for treatment of malignant brain tumors. J Neurointerv Surg. 2022;14:533–8.
Li C, Zhao H, Wang B. Mesenchymal stem/stromal cells: Developmental origin, tumorigenesis and translational cancer therapeutics. Transl Oncol. 2021;14: 100948.
Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, Li L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14:136.
Tang L, He S, Yin Y, Liu H, Hu J, Cheng J, Wang W. Combination of nanomaterials in cell-based drug delivery systems for cancer treatment. Pharmaceutics. 2021;13:1888.
Khalilov R. A comprehensive review of advanced nano-biomaterials in regenerative medicine and drug delivery. Adv Biol Earth Sci. 2023;8:5–18.
Acknowledgements
Not applicable.
Funding
This work was supported in part by grants from the National Science Foundation of China (#82173283 and #82103088), and Foundation of the committee on science and technology of Tian** (#21JCZDJC00230 and 21JCYBJC00190). We acknowledge Editage service for the manuscript language edit.
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: SZ; Methodology: XY and MZ; Validation: YN, JS and YY; Review and editing, SZ; Funding acquisition: SZ.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Medicine Ethics Committee of Tian** Union Medical Center. Written informed consent was obtained from individual or guardian participants.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Competing interests
No potential competing interest are disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. MSC-related gene signature. The gene signatures were extracted from the GOBP_MESENCHYMAL_STEM_CELL_DIFFERENTIATION and GOBP_MESENCHYMAL_STEM_CELL_PROLIFERATION in Molecular Signatures Database.
Additional file 2
. Angiogenesis-related gene signature. The gene signatures were intersection of published papers investigating angiogenesis in ovarian cancer and the HALLMARK_ANGIOGENESIS from Molecular Signatures Database.
Additional file 3
. The immune gene signatures. The gene signatures involved in immunity from 5 literatures and their coefficients.
Additional file 4
. The tumor mutational burden of GSE91061. The tumor mutation burden of GSE91061.
Additional file 5
. The primer sequence. The forward and reverse primer sequence of PER1, AKAP12 and MMP17.
Additional file 6
. Protocol for reverse transcription and real-time polymerase chain reaction. Details for reverse transcription and real-time polymerase chain reaction.
Additional file 7
. Protocol for immune-histochemistry. Details for immune-histochemistry.
Additional file 8
. Grou** of samples in TCGA according to MSC score. The normalized enrichment scores and group information of each sample in TCGA.
Additional file 9
. GO enrichment analysis between low and high MSC score group. GO enrichment analysis of DEGs between groups with low and high MSC scores.
Additional file 10
. KEGG pathway analysis between low and high MSC score group. KEGG pathway analysis of DEGs between groups with low and high MSC scores.
Additional file 11
. Genes related to prognosis in brown module. Prognostic genes in module correlated with MSC score.
Additional file 12
. Genes in the MSC score related prognostic model. Multivariate Cox regression analysis was conducted to identify MSC-score-related genes associated with OS and three genes (MMP17, AKAP12, andPER1) were used to construct the prognostic model.
Additional file 13
. C-index of TCGA cohort, GEO cohorts and nomogram. Standard error, minimum value, maximum value and P value of C-index of TCGA cohort, GEO cohorts and nomogram.
Additional file 14
. Difference between staining index of PER1, AKAP12 and MMP17 and age among SOC samples. Difference between staining index of PER1, AKAP12 and MMP17 and age among SOC samples via Mann-Whitney U test.
Additional file 15
. Difference between staining index of PER1, AKAP12 and MMP17 and tumor size among SOC samples. Difference between staining index of PER1, AKAP12 and MMP17 and tumor size among SOC samples via Mann-Whitney U test.
Additional file 16:
Figure S1. Estimation of the best cutoff value for the MSC scores determined by the X-tile software. The interface of estimation of the best cutoff value for the MSC scores in TCGA-OV cohort by the X-tile software. Figure S2. Identification of the MSC-score-related gene set. (A) Clustering dendrograms to reject outliers. (B) Scale-free network confirmation with connectivity value k. (C) Module dendrogram and (D) gene dendrogram before and after combination of modules with similar expression patterns. Figure S3. Gene expression heatmap, risk scores and OS time distribution Gene expression heatmap, risk scores and OS time distribution of TCGA and GEO cohorts, and ROC curve of GEO datasets. Figure S4. Immunity inhibition factors and genetic characteristics in different MSC risk group. The level of immunity inhibition factors and genetic characteristics in different MSC risk group, which P value was more than 0.05. Figure S5. Estimation of the best cutoff value for the GSE19061 risk scores determined by X-tile software. The interface of estimation of the best cutoff value for the GSE19061 risk scores determined by X-tile software. Figure S6. Kaplan-Meier analysis of other biomarkers of GSE91061. To evaluate the predictive power of other biomarkers, PD-L1 and PD-1 expression status, 14 kinds of tumor-infiltrating lymphocytes, mutational burden, and immune gene signatures were correlation with the survival. Figure S7. Correlation between the abundance score of biomarkers and response to immunotherapy. The scores of other biomarkers in different response groups of GSE19061 and the proportion of patients with response to anti-PD-1 immunotherapy in different biomarker level groups. Figure S8. Evaluation of the prognostic model in the antiangiogenesis dataset and construction of the nomogram. Evaluation of the prognostic model in the antiangiogenesis dataset and conformity between nomogram prediction and actual observation in terms of the 1-(C), 3-(D), and 5-(E)year survival rates. Figure S9. Comparison of risk score in different FIGO stage, which means the condition on metastasis of SOC. Comparison of risk score in different FIGO stage, which means the condition on metastasis of SOC, in TCGA cohort and GSE14764 and GSE53963.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, X., Zheng, M., Ning, Y. et al. Prognostic risk factors of serous ovarian carcinoma based on mesenchymal stem cell phenotype and guidance for therapeutic efficacy. J Transl Med 21, 456 (2023). https://doi.org/10.1186/s12967-023-04284-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04284-3