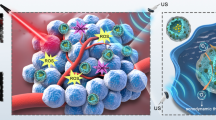
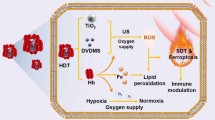
Abstract
Background
Hypoxia-activated prodrug (HAP) is a promising candidate for highly tumor-specific chemotherapy. However, the oxygenation heterogeneity and dense extracellular matrix (ECM) of tumor, as well as the potential resistance to chemotherapy, have severely impeded the resulting overall efficacy of HAP.
Results
A HAP potentiating strategy is proposed based on ultrasound responsive nanodroplets (PTP@PLGA), which is composed of protoporphyrin (PpIX), perfluoropropane (PFP) and a typical HAP, tirapazamine (TPZ). The intense vaporization of PFP upon ultrasound irradiation can magnify the sonomechanical effect, which loosens the ECM to promote the penetration of TPZ into the deep hypoxic region. Meanwhile, the PpIX enabled sonodynamic effect can further reduce the oxygen level, thus activating the TPZ in the relatively normoxic region as well. Surprisingly, abovementioned ultrasound effect also results in the downregulation of the stemness of cancer cells, which is highly associated with drug-refractoriness.
Conclusions
This work manifests an ideal example of ultrasound-based nanotechnology for potentiating HAP and also reveals the potential acoustic effect of intervening cancer stem-like cells.
Graphical Abstract

Similar content being viewed by others
Introduction
Hypoxia is recognized as a common feature of most solid malignant tumors, which has been widely explored as a typical endogenous target for develo** tumor-specific therapies in recent years [1D, The C, N, O elements evenly distributed in the whole PTP@PLGA and the F element mainly located in the core of PTP@PLGA, which demonstrated the successful encapsulation of PFP. The dynamic light scattering results show that the average hydrodynamic sizes (AHS) of PP@PLGA and PTP@PLGA are about 318.9 ± 105.2 nm (PDI = 0.325) and 302 ± 88.06 nm (PDI = 0.258) respectively. In comparison, without the PFP core, TP@PLGA has obviously smaller AHS of 178 ± 60.55 nm (PDI = 0.137) (Fig. 1E). Besides, the average diameter of PTP@PLGA maintained a relatively stable nanoscale and showed a slight increase from 322.5 to 418.2 nm without obvious change of appearance within a 5-day duration (Figure S1). And due to the same surface composition (PLGA-PVA shell), the mean zeta potentials of TP@PLGA, PP@PLGA and PTP@PLGA are approximately equal (Fig. 1F). Furthermore, the successful encapsulation of PpIX and TPZ, which displayed the characteristic absorbance peak at 500 nm and 265 nm respectively, was confirmed by the UV-vis spectra for PP@PLGA, TP@PLGA and PTP@PLGA (Fig. 1G). Based on the established calibration (Figure S2 and Figure S3), the loading efficiency and content of TPZ in PTP@PLGA were calculated to be 26.1 ± 1.96% and 4.5 ± 0.3% respectively, which were both lower than those of PpIX (91.5 ± 1.75% and 7.8 ± 0.1%). Moreover, as shown in Fig. 1H, Figure S4 and Figure S5, comparing to that of free PpIX, no obvious absorbance decrease was observed for PTP@PLGA in 7 days whether kee** it in dark condition or not, indicating the better stability of the encapsulated PpIX within PTP@PLGA.
Preparation and characterization of the nanodroplets. (A) Synthetic procedures of PTP@PLGA via a three-step emulsion method. (B) SEM image and (C) TEM image of PTP@PLGA. (D) Element map**s of PTP@PLGA. Scale bar = 100 nm. (E) Dynamic light scattering results of TP@PLGA, PP@PLGA and PTP@PLGA. (F) ζ-potential of TP@PLGA, PP@PLGA and PTP@PLGA. (G) UV-vis spectra of TPZ, PpIX, PP@PLGA, TP@PLGA and PTP@PLGA. (H) UV-vis spectra of PTP@PLGA in the dark for 7 days
Concurrent ultrasound-induced vaporization and drug release
The boiling point of PFP is 29 ℃ under standard pressure, which could be elevated when PFP is emulsified and encapsulated in nanodroplets due to the additional Laplace pressure [40]. For PTP@PLGA, as shown in the optical micrographs (Fig. 2A), only at temperatures above 42 ℃, the encapsulated PFP could vaporize, generating microbubbles with larger diameters. In addition to the thermal stimulation, the vaporization of PFP-based nanodroplets could also be triggered via ultrasound due to the applied negative peak pressure, namely acoustic droplet vaporization (ADV). In vitro ultrasound imaging (B-mode and contrast-mode) were further conducted to confirm the ADV process for PTP@PLGA, since the generated microbubbles could act as ultrasound contrast agents. As shown in Fig. 2B, before ultrasound irradiation, both TP@PLGA and PTP@PLGA exhibited no obvious echo changes comparing to PBS due to their nanoscale size. After ultrasound irradiation (1 W/cm2 for 30 s), the ultrasound images of PBS remained almost the same. As expected, significantly brighter images were captured in both B-mode and contrast-mode ultrasound imaging for PTP@PLGA, confirming the generation of microbubbles via ADV. Comparatively, without the emulsified PFP, TP@PLGA only exhibited slight echo change, which was also confirmed by the quantitative results (Fig. 2C and D).
Then, the ADV effect on entrapped TPZ release was further investigated via UV-vis spectra. Calculated based on Lambert-Beer law, without ultrasound stimulation, TP@PLGA and PTP@PLGA exhibited only 15.2% and 21.7% release of TPZ in 250 min respectively. As expected, under periodic ultrasound stimulation, the release ratio of TPZ for PTP@PLGA steeply rose up to 61% within 250 min, which was obviously higher than that of TP@PLAG (Fig. 2E). Theoretically, ultrasound itself can accelerate the diffusion rate of drug via its thermal or mechanical effect, which has been commonly reported [41,3A, three peaks with the same intensity were manifested in the coexistence of PTP@PLGA and US, which suggested that PTP@PLGA could produce 1O2 triggered by US. Additionally, the capability of PTP@PLGA to generate .OH was also assessed. As shown in Fig. 3B, both the US and PTP@PLGA + US groups displayed the representative .OH signals with an intensity ration of 1:2:2:1. Then the 1O2 production was further evaluated by using DPBF as trap** reagent, which can react with 1O2 to form an endoperoxide, showing a decrease at 410 nm in the UV-vis spectrum. As shown in Fig. 3C and D, when incubated with PTP@PLGA under ultrasound irradiation, the characteristic absorption peak at 410 nm of DPBF decayed gradually with the prolonging of duration and increasing of power intensity, showing a typical time and intensity dependent manner. Thus, for PTP@PLGA, in addition to ADV, the applied ultrasound can also activate O2, yielding 1O2 via PpIX sensitization. With the same content of PpIX, PP@PLGA and TP@PLGA (Figure S6) exhibited the similar phenomenon under ultrasound irradiation. And no significant difference was found among the above three PpIX-loaded nanocarriers, regarding the DPBF oxidation experiment via sonodynamic effect. Meanwhile, during ultrasound irradiation, the oxygen level was also measured via a portable dissolved oxygen meter. Due to the efficient sonodynamic effect of PTP@PLGA, the dissolved oxygen could be effectively converted to 1O2. Thus, the concentration of the dissolved oxygen decreased under ultrasound irradiation, exhibiting a similar time and power dependent manner as shown in Fig. 3E and F. With the same content of PpIX, TP@PLGA showed negligible difference on the change of oxygen concentration in comparison to PTP@PLGA (Figure S7). Thus, it is believed that the PpIX mediated sonodynamic effect can form hypoxic environment for the further activation of TPZ via oxygen depletion.
Furthermore, intracellular ROS generation via sonodynamic effect was evaluated using DCFH-DA as the ROS indicating agent, which could be converted to dichlorofluorescein with green fluorescence in the presence of 1O2. As show in the confocal laser scanning microscope (CLSM) images (Fig. 3G), DCFH-DA stained 4T1 cells were divided into 4 groups (control, US, PTP@PLGA, PTP@PLGA + US) for different treatments, in which only the PTP@PLGA + US group exhibited obvious green fluorescence covering the entire cells. The corresponding quantitative analysis via flow cytometry further confirmed that the PTP@PLGA + US group yielded the strongest green fluorescence among all the treated groups (Fig. 3H). With the same content of PpIX, the similar results were also found for both PP@PLGA and TP@PLGA (Figure S8). And the SDT-induced ROS generation and exacerbated hypoxia was further evaluated by Hypoxia/Oxidative Stress Detection probe. As presented in Fig. 3I, the 4T1 cells in the control, US and PTP@PLGA groups showed no obvious fluorescence signal. While intensive red (hypoxia) and green fluorescence (ROS) was observed in the PTP@PLGA + US group due to the ROS generation and SDT-induced hypoxia. Thus, as expected, the PpIX-entrapped nanocarriers could be endocytosed in the 4T1 cells, rendering the intracellular 1O2 generation under ultrasound irradiation.
Sonodynamic effect and the consequent oxygen consumption. ESR spectra of (A) 1O2 and (B) .OH generation in control, US, PTP@PLGA and PTP@PLGA + US group. The absorption spectra of DPBF incubated with PTP@PLGA under ultrasound irradiation with (C) different durations (1 W/cm2 for 30, 60 and 120 s) or with (D) different power intensities (0.5, 1 and 1.5 W/cm2 for 60 s). The change of oxygen concentration in PP@PLGA and PTP@PLGA under ultrasound irradiation with (E) different durations (1 W/cm2 for 30, 60, 120 and 180 s) or with (F) different power intensities (0.5, 1 and 1.5 W/cm2 for 60 s). (G) CLSM images of DCFH-DA stained 4T1 cells with different treatments (control, US, PTP@PLGA, PTP@PLGA + US). Scale bar = 40 μm. (H) The corresponding flow cytometry quantitative analysis of ROS generation in 4T1 cells with different treatments. (I). CLSM images of 4T1 cells stained by Hypoxia/Oxidative Stress Detection probe under different treatments (control, US, PTP@PLGA, PTP@PLGA + US). Scale bar = 40 μm
Synergetic therapy in cell level
As designed and shown in Fig. 4A, PP@PLGA, TP@PLGA and PTP@PLGA possessed diverse ultrasound-responsive behaviors, which were further evaluated and compared on therapeutic efficacy for 4T1 cells to reveal the proposed synergetic effect of PTP@PLGA.
As shown in Fig. 4B and Figure S9, without US irradiation, TP@PLGA, PP@PLGA and PTP@PLGA nanodroplets with varied concentrations of PpIX and TPZ all exhibit negligible cytotoxicity to 4T1 cells and 3T3 fibroblast cells at normoxic condition, indicating their excellent biocompatibility. Furthermore, the therapeutic effect of PTP@PLGA under US irradiation was also evaluated in the cell level. As shown in Fig. 4C, under two power output of US (1 W/cm2 and 2 W/cm2, 3 min), all PP@PLGA, TP@PLGA and PTP@PLGA show significant cytotoxicity, in which, as expected, the PTP@PLGA induces highest cytotoxicity. Thus, it can be inferred that for PTP@PLGA, in addition to the 1O2 generated via sonodynamic effect, the simultaneous oxygen consumption can further activate the hypoxia-sensitive TPZ, resulting in synergistic therapeutic effect, which is superior to PP@PLGA with sonodynamic effect alone. Meanwhile, the cytotoxicity induced by TP@PLGA + US is also lower than that by PTP@PLGA + US since less TPZ could be released from TP@PLGA nanodroplets without the effect of PFP vaporization. Furthermore, the therapeutic outcome was also directly observed via CLSM, where the dead cells were stained with PI (red fluorescence) and the living cells were stained with calcein-AM (green fluorescence). As shown in Fig. 4D, red fluorescence was found in the CLSM images of multiple groups, in which the PTP@PLGA + US (1 W/cm2 for 3 min) treated group exhibited the most significant red fluorescence, indicating the massive cell death under such treatment due to the synergistic therapeutic effect. The flow cytometric assay was also conducted to investigate such ultrasound-mediated therapeutic effect. As shown in Fig. 4E, compared to other treated groups (control, US), PP@PLGA, TP@PLGA and PTP@PLGA could mainly induce apoptosis of 4T1 cells under US irradiation (1 W/cm2 for 3 min), in which the proportion of late apoptosis is higher than that of the early apoptosis. Interestingly, the ratio of early and late apoptosis of 4T1 cells in PTP@PLGA + US treated group is almost the same with that in PP@PLGA + US treated group. As expected, the total proportion of apoptosis that PTP@PLGA caused is significantly higher than that of PP@PLGA under US irradiation, which is consistent with the above CCK-8 and CLSM results. Thus, the US-mediated synergistic therapeutic effect of PTP@PLGA has been proved as a viable way to kill cancer cells via inducing apoptosis.
In vitro ultrasound effect on cancer stem-like cells
As a small portion of cancer cells, the cancer stem-like cells (CSCs) are believed to exhibit higher chemoresistance. Thus, in order to further evaluate the ultrasound effect on CSCs, PP@PLGA was chosen to co-incubated with CSCs under ultrasound stimulation, which were enriched from 4T1 cells in a serum-free culture according to the reported method [55]. Briefly, DPBF in DMF (10 µL, 10 mM) was added to 2 mL of PP@PLGA, TP@PLGA and PTP@PLGA with the same concentration of PpIX (20 µg/mL), respectively. Then, the solution was exposed to ultrasound with different power intensities (0.5, 1 and 1.5 W/cm2 for 60 s) or different durations (1 W/cm2 for 30, 60 and 120 s), respectively. The absorption intensity of DPBF at 410 nm was measured by UV-vis spectrometer. Meanwhile, the oxygen level before and after ultrasound irradiation was also measured using a portable dissolved oxygen meter (JPB-607 A). The PP@PLGA, TP@PLGA and PTP@PLGA nanodroplets (PpIX, 20 µg/mL) were dissolved in degassed water and then exposed to ultrasound with different power intensities (60 s, 0.5, 1 and 1.5 W/cm2) or different durations (1 W/cm2, 30, 60, 120 and 180 s), respectively. And DCFH-DA was employed to evaluate the intracellular singlet oxygen generation. 4T1 cells were co-incubated with PBS, PP@PLGA, TP@PLGA and PTP@PLGA with the same concentration of PpIX (20 µg/mL) for 4 h, respectively. Then, the above 4T1 cells were incubated with DCFH-DA for 0.5 h, which were subsequently irradiated by ultrasound (1 W/cm2 for 180 s). After incubation for another 0.5 h, these cells were collected for analysis via flow cytometry (CytomicsTM FC500 cytometer, Beckman Coulter) and confocal laser scanning microscope (FV 1000, Olympus). The intracellular ROS and hypoxia generation were further assessed by using hypoxia/Oxidative stress detection kit. The 4T1 cells divided into four groups: control, US, PTP@PLGA and PTP@PLGA + US. These cells were treated with PBS or PTP@PLGA for 4 h and subsequently irradiated with or without ultrasound. Then the cells in different treatment groups were imaged by CLSM.
Cell culture and animal model 4T1 cells were cultured in PRMI 1640 medium with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 ℃ in atmosphere with 5% CO2. For the formation of mammospheres, 4T1 cells were seeded into ultra-low attachment six-well plates (Corning, USA) and incubated with DMEM/F12 medium supplemented with B-27, 20 ng/mL of fibroblast growth factors-basic, 20 ng/mL epidermal growth factor, and 5 µg/mL insulin for about 10 days [14, 19].
All animal studies were conducted under a protocol approved by the Animal Ethics Committee of Fudan University (2019 JS-144). And the 4T1 breast cancer xenografts were formed by subcutaneous administration of 1 × 106 4T1 cells in 100 µL of serum-free medium into the back of the female BALB/c mice. And all experiments were carried out when the tumor volumes reached about 80 mm3.
Synergetic therapy in cell level
To evaluate the cytotoxicity of TP@PLGA, PP@PLGA and PTP@PLGA nanodroplets, 4T1 cells were incubated with them at different concentrations (the concentration of PpIX is 7.5, 15 and 30 µg/mL) for 24 h. And cell viabilities were determined by CCK-8 assay. To test the therapeutic effects, 4T1 cells were treated with different concentrations of TP@PLGA, PP@PLGA and PTP@PLGA nanodroplets for 4 h, which were then exposed to ultrasound irradiation (1–2 W/cm2) for 3 min. After another 24 h incubation, the cell viabilities were determined by CCK-8 kit, flow cytometry and further assessed by confocal fluorescence microscopy after co-staining with CAM (2 µM) and PI (4 µM) [54].
In vitro stem-like feature evaluation
The stem-like feature of cancer cells, including the transcription factor (SOX2, NANOG and OCT4) and acetaldehyde dehydrogenase (ALDH), was evaluated for adherent 4T1 cells and mammospheres (cancer stem-like cells) under different treatments (PP@PLGA, US and PP@PLGA + US, PpIX, 20 µg/mL), US power output: 1 W/ cm2 for 3 min), which were determined using the real-time polymerase chain reaction (PCR) system (Thermo Fisher, America) and the ALDEFLUOR kit via flow cytometry, respectively. The primer sequences of the targeted genes (SOX2, NANOG and OCT4) were shown in Table S1.
In vivo ultrasound imaging and analysis of drug distribution
For in vivo biodistribution of PTP@PLGA, the tumor-bearing mice were randomly divided to two groups: PTP@PLGA and PTP@PLGA + US. The PTP@PLGA were intravenously injected with the same content of PLGA (2 mg/mL) and the ultrasound irradiation (1 W/cm2 for 10 min) was applied at the tumor sites 5 min post injection. The fluorescence images were obtained at different time points (pre, 1, 2, 4, 6, 18, 24, 48 and 72 h) by fluorescence imaging system. At 72 h post injection, the tumor tissues and main organs were collected to evaluate the fluorescence intensities.
For in vivo US imaging, the tumor-bearing mice were randomly divided to three groups and intravenously injected of PBS, TP@PLGA and PTP@PLGA with the same content of PLGA (2 mg/mL), respectively. Then, ultrasound irradiation (1 W/cm2 for 10 min) was applied at the tumor sites 5 min post intravenously injecting. Meanwhile, the B-mode and contrast-mode US (CEUS) images of tumors were captured by ultrasonic instrument (Resona 7, China) and the echo intensities were measured by an US image analyzer (DFY software, Institute of Ultrasound Imaging of Chong Qing Medical University). The in vivo drug release from nanodroplets were observed and analyzed by detecting the fluorescence of PpIX as the fluorescence of TPZ was too weak to be detected. The tumor-bearing mice were randomly divided into four groups for different treatments: (1) TP@PLGA; (2) TP@PLGA + US; (3) PTP@PLGA; (4) PTP@PLGA + US. For group 2 and 4, ultrasound irradiation (1 W/cm2 for 10 min) was applied at the tumor sites of the mice 5 min post i.v. injection of the nanodroplets (PpIX: 9 mg/kg; TPZ: 6 mg/kg). Then, after 24 h, tumors from all the groups were harvested and stained with anti-CD31 antibody and DAPI to label blood vessel and cell nucleus, respectively. And the PpIX distribution was observed by confocal laser scanning microscope.
In vivo oxygen level evaluation in tumor
To assay the sonodynamic effect on hypoxia status in tumors, HIF-1α antibody was used to stain ex vivo tissue for immunostaining analysis. The tumor-bearing mice were randomly divided into three groups (n = 3 per group) for different treatments: (1) saline; (2) US; (3) PTP@PLGA + US. For mice in group 3, US irradiation (1 W/cm2 for 10 min) was applied at the tumor sites of mice 5 min post intravenous (i.v.) injection of nanodroplets (PpIX: 9 mg/kg; TPZ: 6 mg/kg). 24 h later, all the tumors were collected for HIF-1α analysis. The hypoxia status was further evaluated by photoacoustic imaging (Vevo LAZER) by measuring the change of blood oxygen saturation (sO2) at tumor region. The tumor-bearing mice were divided into four groups: (1) Control; (2) US; (3) PTP@PLGA; (4) PTP@PLGA + US. Ultrasound (1.0 W/cm2, 10 min) was applied at the tumor sites of mice 5 min post intravenous (i.v.) injection of PTP@PLGA nanodroplets (PpIX: 9 mg/kg; TPZ: 6 mg/kg). The photoacoustic images of tumor site and quantitative analysis of signal intensities were performed before and after ultrasound irradiation.
In vivo assessment of tumor tissue
The tumor-bearing mice were randomly divided into six groups (n = 3 per group) for different treatments: (1) US1(0.5 W/cm2, 10 min); (2) US2 (1.0 W/cm2, 10 min); (3) US3 (1.5 W/cm2, 10 min); (4) PTP@PLGA + US1; (5) PTP@PLGA + US2; (6) PTP@PLGA + US3. Ultrasound was applied at the tumor sites of mice 5 min post intravenous (i.v.) injection of PTP@PLGA nanodroplets (PpIX: 9 mg/kg; TPZ: 6 mg/kg). Ultrasound shear-wave elastography (SWE) was used to quantitatively evaluate the Young’s elastic modulus of tumor tissue for mice before and after US irradiation. At the third day post treatments, the tumors in each group were collected and evaluated by Gordon-Sweets staining and Masson staining to indicate the changes of ECM structure including reticular fiber and collagen fiber.
In vivo synergetic therapy
The tumor-bearing mice were randomly divided into six groups (n = 6 in each group) for different treatments: (1) control; (2) US only; (3) TPZ (4) PP@PLGA + US; (5) TP@PLGA + US; (6) PTP@PLGA + US, in which saline, TPZ, PP@PLGA, TP@PLGA and PTP@PLGA were all intravenously injected. For group 4, 5 and 6, US irradiation (1 W/cm2 for 10 min) was applied at the tumor sites of mice 5 min post intravenous (i.v.) injection of different nanodroplets (PpIX, 9 mg/kg; TPZ, 6 mg/kg). All the treatments for all the mice in each group were repeated every three days and the therapeutic efficacy was evaluated in 16 days by recording the tumor volumes and body weights of mice in each group. Tumor volume was calculated using the equation: tumor volume = length × (width)2 / 2. After 16 days, tumors and major organs were collected for hematoxylin and eosin (HE) and transferase-mediated deoxyuridine triphosphatebiotin nick end labeling (TUNEL) staining to evaluate the therapeutic outcome.
In vivo assessment of stemness-related factor expression
The stem-like feature of cancer cells, including the surface biomarker (CD24 and CD44), transcription factor (NANOG and SOX2) was evaluated by immunofluorescence and immunohistochemical analysis respectively. The tumor-bearing mice were randomly divided into four groups (n = 3 per group) for different treatments: (1) saline; (2) US; (3) PTP@PLGA; (4) PTP@PLGA + US. For mice in group 4, US irradiation (1 W/cm2 for 10 min) was applied at the tumor sites of mice 5 min post intravenous (i.v.) injection of nanodroplets (PpIX: 9 mg/kg; TPZ: 6 mg/kg). After 3 days, tumors were collected for abovementioned immunohistochemical staining.
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical comparisons between two groups were conducted by Student’s t test. And *p < 0.05, **p < 0.01, ***p < 0.005 and ****p < 0.001 were considered significant.
Data availability
No datasets were generated or analysed during the current study.
References
**g X, Yang F, Shao C, Wei K, **e M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18:157.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410.
Phillips RM. Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs. Cancer Chemother Pharmacol. 2016;77:441–57.
Sharma A, Arambula JF, Koo S, Kumar R, Singh H, Sessler JL, Kim JS. Hypoxia-targeted drug delivery. Chem Soc Rev. 2019;48:771–813.
Yang S, Tang Z, Hu C, Zhang D, Shen N, Yu H, Chen X. Selectively potentiating hypoxia levels by combretastatin A4 nanomedicine: toward highly enhanced hypoxia-activated prodrug tirapazamine therapy for metastatic tumors. Adv Mater. 2019;31:e1805955.
Zhao H, Zhao B, Li L, Ding K, **ao H, Zheng C, Sun L, Zhang Z. Biomimetic decoy inhibits tumor growth and lung metastasis by reversing the drawbacks of sonodynamic therapy. Adv Healthc Mater. 2020;9:e1901335.
Wang H, Li J, Wang Y, Gong X, Xu X, Wang J, Li Y, Sha X, Zhang Z. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J Control Release. 2020;319:25–45.
Sahu A, Kwon I, Tae G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials. 2020;228:119578.
Kumari R, Sunil D, Ningthoujam RS. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J Control Release. 2020;319:135–56.
Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92.
Dewhirst MW, Secomb TW. Transport of drugs from blood vessels to tumour tissue. Nat Rev Cancer. 2017;17:738–50.
Mai TT, Hamai A, Hienzsch A, Caneque T, Muller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. 2017;9:1025–33.
Shen S, Lin S, Chen Y, Zhang Y, He Y, Xu X, Feng Y, Lu Y, Mo R. Combating cancer stem-like cell-derived resistance to anticancer protein by liposome-mediated acclimatization strategy. Nano Lett. 2022;22:2419–28.
Chen Q, Deng B, Luo Q, Song G. Deep tumor-penetrated nanosystem eliminates cancer stem cell for highly efficient liver cancer therapy. Chem Eng J. 2021;421:127874.
Liu M, Wang L, Zheng X, Liu S, **e Z. Hypoxia-triggered nanoscale metal-organic frameworks for enhanced anticancer activity. ACS Appl Mater Interfaces. 2018;10:24638–47.
Shao Y, Liu B, Di Z, Zhang G, Sun LD, Li L, Yan CH. Engineering of upconverted metal-organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors. J Am Chem Soc. 2020;142:3939–46.
Zhang MK, Li CX, Wang SB, Liu T, Song XL, Yang XQ, Feng J, Zhang XZ. Tumor starvation induced spatiotemporal control over chemotherapy for synergistic therapy. Small. 2018;14:e1803602.
Zhang R, Feng L, Dong Z, Wang L, Liang C, Chen J, Ma Q, Zhang R, Chen Q, Wang Y, Liu Z. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia-activated therapy. Biomaterials. 2018;162:123–31.
Pan Y, Ma X, Liu C, **ng J, Zhou S, Parshad B, Schwerdtle T, Li W, Wu A, Haag R. Retinoic acid-loaded dendritic polyglycerol-conjugated gold nanostars for targeted photothermal therapy in breast cancer stem cells. ACS Nano. 2021;15:15069–84.
Shen S, Xu X, Lin S, Zhang Y, Liu H, Zhang C, Mo R. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat Nanotechnol. 2021;16:104–13.
Li Z, Shan X, Chen Z, Gao N, Zeng W, Zeng X, Mei L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv Sci. 2020;8:2002589.
Wang Y, **e Y, Li J, Peng Z-H, Sheinin Y, Zhou J, Oupický D. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano. 2017;11:2227–38.
Ihsanullah KM, Kumar BN, Zhao Y, Muhammad H, Liu Y, Wang L, Liu H, Jiang W. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomaterials. 2020;245:119982.
Ahsan SM, Rao CM, Ahmad MF. Nanoparticle-protein interaction: the significance and role of protein corona. Adv Exp Med Biol. 2018;1048:175–98.
Pareek V, Bhargava A, Bhanot V, Gupta R, Jain N, Panwar J. Formation and characterization of protein corona around nanoparticles: a review. J Nanosci Nanotechnol. 2018;18:6653–70.
Yue W, Chen L, Yu L, Zhou B, Yin H, Ren W, Liu C, Guo L, Zhang Y, Sun L et al. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat Commun. 2019, 10:2025.
**ang H, Chen Y. Energy-converting nanomedicine. Small. 2019;15:e1805339.
Xu Q, Zhan G, Zhang Z, Yong T, Yang X, Gan L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics. 2021;11:1937–52.
Zhou L-Q, Li P, Cui X-W, Dietrich CF. Ultrasound nanotheranostics in fighting cancer: advances and prospects. Cancer Lett. 2020;470:204–19.
Lin X, Song J, Chen X. Ultrasound-activated sensitizers and applications. Angew Chem Int Ed Engl. 2020;59:14212–33.
Wu P, Sun Y, Dong W, Zhou H, Guo S, Zhang L, Wang X, Wan M, Zong Y. Enhanced anti-tumor efficacy of hyaluronic acid modified nanocomposites combined with sonochemotherapy against subcutaneous and metastatic breast tumors. Nanoscale. 2019;11:11470–83.
Mannaris C, Yang C, Carugo D, Owen J, Lee JY, Nwokeoha S, Seth A, Teo BM. Acoustically responsive polydopamine nanodroplets: a novel theranostic agent. Ultrason Sonochem. 2020;60:104782.
Li G, Wang S, Deng D, **ao Z, Dong Z, Wang Z, Lei Q, Gao S, Huang G, Zhang E, et al. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer-conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano. 2020;14:1586–99.
Tang H, Zheng Y, Chen Y. Materials chemistry of nanoultrasonic biomedicine. Adv Mater 2017, 29.
Cao B, Lyu X, Wang C, Lu S, **ng D, Hu X. Rational collaborative ablation of bacterial biofilms ignited by physical cavitation and concurrent deep antibiotic release. Biomaterials. 2020;262:120341.
Wang Z, Zhan M, Li W, Chu C, **ng D, Lu S, Hu X. Photoacoustic cavitation-ignited reactive oxygen species to amplify peroxynitrite burst by photosensitization-free polymeric nanocapsules. Angew Chem Int Ed Engl. 2021;60:4720–31.
Wang Z, Zhan M, Hu X. Pulsed laser excited Photoacoustic Effect for Disease diagnosis and therapy. Chemistry. 2022;28:e202200042.
Fadera S, Chen PY, Liu HL, Lee IC. Induction therapy of retinoic acid with a temozolomide-loaded gold nanoparticle-associated ultrasound effect on glioblastoma cancer stem-like colonies. ACS Appl Mater Interfaces. 2021;13:32845–55.
Guo L, Zheng P, Fan H, Wang H, Xu W, Zhou W. Ultrasound reverses chemoresistance in breast cancer stem cell like cells by altering ABCG2 expression. Biosci Rep 2017, 37.
Deng L, Cai X, Sheng D, Yang Y, Strohm EM, Wang Z, Ran H, Wang D, Zheng Y, Li P, et al. A laser-activated biocompatible theranostic nanoagent for targeted multimodal imaging and photothermal therapy. Theranostics. 2017;7:4410–23.
Eggen S, Fagerland SM, Morch Y, Hansen R, Sovik K, Berg S, Furu H, Bohn AD, Lilledahl MB, Angelsen A, et al. Ultrasound-enhanced drug delivery in prostate cancer xenografts by nanoparticles stabilizing microbubbles. J Control Release. 2014;187:39–49.
Ding J, Chen J, Gao L, Jiang Z, Zhang Y, Li M, **ao Q, Lee SS, Chen X. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29:100800.
Goins B, Phillips WT, Bao A. Strategies for improving the intratumoral distribution of liposomal drugs in cancer therapy. Expert Opin Drug Deliv. 2016;13:873–89.
Hadinger KP, Marshalek JP, Sheeran PS, Dayton PA, Matsunaga TO. Optimization of phase-change contrast agents for targeting MDA-MB-231 breast cancer cells. Ultrasound Med Biol. 2018;44:2728–38.
Huang Y, Vezeridis AM, Wang J, Wang Z, Thompson M, Mattrey RF, Gianneschi NC. Polymer-stabilized perfluorobutane nanodroplets for ultrasound imaging agents. J Am Chem Soc. 2017;139:15–8.
Fan CH, Lin YT, Ho YJ, Yeh CK. Spatial-temporal cellular bioeffects from acoustic droplet vaporization. Theranostics. 2018;8:5731–43.
Morgan CA, Parajuli B, Buchman CD, Dria K, Hurley TD. N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem Biol Interact. 2015;234:18–28.
Song S, Ma D, Xu L, Wang Q, Liu L, Tong X, Yan H. Low-intensity pulsed ultrasound-generated singlet oxygen induces telomere damage leading to glioma stem cell awakening from quiescence. iScience. 2022;25:103558.
Zhang Q, ** H, Chen L, Chen Q, He Y, Yang Y, Ma S, **ao S, ** F, Luo Q, Liu J. Effect of ultrasound combined with microbubble therapy on interstitial fluid pressure and VX2 tumor structure in rabbit. Front Pharmacol. 2019;10:716.
Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303–29.
Liu G, Zhang MK, He Y, Li XR, Wang ZL. Shear wave elasticity of breast lesions: would it be correlated with the extracellular matrix components? Gland Surg. 2019;8:399–406.
Mohammadabadi A, Huynh RN, Wadajkar AS, Lapidus RG, Kim AJ, Raub CB, Frenkel V. Pulsed focused ultrasound lowers interstitial fluid pressure and increases nanoparticle delivery and penetration in head and neck squamous cell carcinoma xenograft tumors. Phys Med Biol. 2020;65:125017.
Lovmo MK, Yemane PT, Bjorkoy A, Hansen R, Cleveland RO, Angelsen BA, de Lange Davies C. Effect of acoustic radiation force on displacement of nanoparticles in collagen gels. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68:416–31.
Sheng D, Liu T, Deng L, Zhang L, Li X, Xu J, Hao L, Li P, Ran H, Chen H, Wang Z. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials. 2018;165:1–13.
Gong F, Cheng L, Yang N, Betzer O, Feng L, Zhou Q, Li Y, Chen R, Popovtzer R, Liu Z. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv Mater. 2019;31:1900730.
Acknowledgements
Not applicable.
Funding
This work was financially supported by National Natural Science Foundation of China (No. 32030061, 81901760, and 81830058), China Postdoctoral Science Foundation (No. 2023M732242) and Shanghai Post-doctoral Excellence Program of Shanghai Municipal Human Resources and Social Security Bureau (No. 2022328).
Author information
Authors and Affiliations
Contributions
Tianzhi Liu, Hangrong Chen and Cai Chang conceived and designed the experiment. Sheng Danli, Lang Qian, Jufeng Chen and Yi Wei performed the experiment and analyzed the data. Sheng Danli and Tianzhi Liu wrote and revised the manuscript. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal studies were conducted under a protocol approved by the Animal Ethics Committee of Fudan University (2019 JS-144).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sheng, D., Liu, T., Qian, L. et al. Sonodynamic and sonomechanical effect on cellular stemness and extracellular physicochemical environment to potentiate chemotherapy. J Nanobiotechnol 22, 358 (2024). https://doi.org/10.1186/s12951-024-02623-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02623-0