Abstract
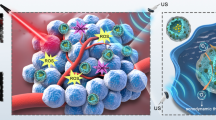
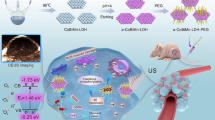
Sonodynamic therapy (SDT) has attracted great interest in the field of cancer therapy because of its non-invasiveness, deep penetration, and spatiotemporal controllability. However, the sonodynamic effect is severely hindered by hypoxia and a high glutathione (GSH) level in tumor microenvironment. In this work, a new type of nanohybrid sonosensitizer is designed, which incorporates several prominent advantages of organic, inorganic, and natural sonosensitizers for imaging-guided SDT and ferroptosis induction. As an endogenous transporter, hemoglobin (Hb) has dual functions of oxygen and iron supplement. Apparently, Hb can transport iron to the tumor site for Fe-dependent ferroptosis, yet this oxygen carrier is able to enhance the sensitivity of tumor cells to oxygen-driven SDT. We innovatively hybridized Hb, sinoporphyrin sodium (DVDMS), and titanium dioxide (TiO2) to prepare an integrated nano-oxygen carrier (designated as HDT) for multifarious cancer theranostics, which not only attained satisfactory sonosensitization of DVDMS/TiO2 but also achieved oxygen-boosted SDT and potent ferroptosis via Hb. Under ultrasound irradiation, the oxygenation of HDT markedly amplified the generation of reactive oxygen species (ROS) in hypoxic tumors, promoted the valence transition of Hb to accelerate Fenton reaction, and led to ferroptosis. This strategy virtually eradicated tumors in situ and exhibited immunoregulatory potential for augmenting anti-tumor effects in vivo and in vitro through ROS formation, O2 self-supplement, GSH depletion, and lipid peroxidation. Collectively, HDT demonstrated excellent dual-modal imaging performance, providing a valuable platform for photoacoustic/fluorescence-guided SDT and ferroptosis induction in tumor cells.

Similar content being viewed by others
References
Pan, X. T.; Wang, H. Y.; Wang, S. H.; Sun, X.; Wang, L. J.; Wang, W. W.; Shen, H. Y.; Liu, H. Y. Sonodynamic therapy (SDT): A novel strategy for cancer nanotheranostics. Sci. China Life Sci.2018, 61, 415–426.
McHale, A. P.; Callan, J. F.; Nomikou, N.; Fowley, C.; Callan, B. Sonodynamic therapy: Concept, mechanism and application to cancer treatment. In Therapeutic Ultrasound; Escoffre, J. M.; Bouakaz, A., Eds.; Springer: Cham, 2016; pp 429–450.
Wood, A. K. W.; Sehgal, C. M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol.2015, 41, 905–928.
Qian, X. Q.; Zheng, Y. Y.; Chen, Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mater.2016, 28, 8097–8129.
Son, S.; Kim, J. H.; Wang, X. W.; Zhang, C. L.; Yoon, S. A.; Shin, J.; Sharma, A.; Lee, M. H.; Cheng, L.; Wu, J. S. et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev.2020, 49, 3244–3261.
Lin, X. H.; Song, J. B.; Chen, X. Y.; Yang, H. H. Ultrasound-activated sensitizers and applications. Angew. Chem., Int. Ed.2020, 59, 14212–14233.
Liang, C.; Zhang, X. L.; Wang, Z. C.; Wang, W. J.; Yang, M. S.; Dong, X. C. Organic/inorganic nanohybrids rejuvenate photodynamic cancer therapy. J. Mater. Chem. B2020, 8, 4748–4763.
Zhao, N. N.; Yan, L. M.; Zhao, X. Y.; Chen, X. Y.; Li, A. H.; Zheng, D.; Zhou, X.; Dai, X. G.; Xu, F. J. Versatile types of organic/inorganic nanohybrids: From strategic design to biomedical applications. Chem. Rev.2019, 119, 1666–1762.
Wang, X. W.; Zhong, X. Y.; Gong, F.; Chao, Y.; Cheng, L. Newly developed strategies for improving sonodynamic therapy. Mater. Horiz.2020, 7, 2028–2046.
Chen, X.; Li, J. B.; Kang, R.; Klionsky, D. J.; Tang, D. L. Ferroptosis: Machinery and regulation. Autophagy2021, 17, 2054–2081.
Nguyen, T. T.; Asakura, Y.; Koda, S.; Yasuda, K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrason. Sonochem.2017, 39, 301–306.
Dixon, S. J.; Lemberg, K. M.; Lamprecht, M. R.; Skouta, R.; Zaitsev, E. M.; Gleason, C. E.; Patel, D. N.; Bauer, A. J.; Cantley, A. M.; Yang, W. S. et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell2012, 149, 1060–1072.
**e, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell. Death Differ.2016, 23, 369–379.
Liang, X. L.; Chen, M.; Bhattarai, P.; Hameed, S.; Tang, Y. D.; Dai, Z. F. Complementing cancer photodynamic therapy with ferroptosis through iron oxide loaded porphyrin-grafted lipid nanoparticles. ACS Nano2021, 15, 20164–20180.
Pan, W. L.; Tan, Y.; Meng, W.; Huang, N. H.; Zhao, Y. B.; Yu, Z. Q.; Huang, Z.; Zhang, W. H.; Sun, B.; Chen, J. X. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials2022, 283, 121449.
Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q. C.; Yu, X. J.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol.2020, 13, 110.
Xu, H. J.; Ye, D.; Ren, M. L.; Zhang, H. Y.; Bi, F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med.2021, 27, 856–867.
Song, R. D.; Li, T. L.; Ye, J. Y.; Sun, F.; Hou, B.; Saeed, M.; Gao, J.; Wang, Y. J.; Zhu, Q. W.; Xu, Z. A. et al. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv. Mater.2021, 33, 2101155.
Tsuchida, E.; Sou, K.; Nakagawa, A.; Sakai, H.; Komatsu, T.; Kobayashi, K. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjugate Chem.2009, 20, 1419–1440.
Xu, T.; Ma, Y. Y.; Yuan, Q. L.; Hu, H. X.; Hu, X. K.; Qian, Z. Y.; Rolle, J. K.; Gu, Y. Q.; Li, S. W. Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano2020, 14, 3414–3425.
Zhou, A. W.; Fang, T. L.; Chen, K. R.; Xu, Y. R.; Chen, Z.; Ning, X. H. Biomimetic activator of sonodynamic ferroptosis amplifies inherent peroxidation for improving the treatment of breast cancer. Small2022, 18, 2106568.
Yuan, M.; Liang, S.; Zhou, Y.; **ao, X.; Liu, B.; Yang, C. Z.; Ma, P. A.; Cheng, Z. Y.; Lin, J. A robust oxygen-carrying hemoglobin-based natural sonosensitizer for sonodynamic cancer therapy. Nano Lett.2021, 21, 6042–6050.
Wang, Y. H.; Sun, Y.; Liu, S. P.; Zhi, L. J.; Wang, X. B. Preparation of sonoactivated TiO2-DVDMS nanocomposite for enhanced antibacterial activity. Ultrason. Sonochem.2020, 63, 104968.
Sharma, A.; Arambula, J. F.; Koo, S.; Kumar, R.; Singh, H.; Sessler, J. L.; Kim, J. S. Hypoxia-targeted drug delivery. Chem. Soc. Rev.2019, 48, 771–813.
Sung, Y. C.; **, P. R.; Chu, L. A.; Hsu, F. F.; Wang, M. R.; Chang, C. C.; Chiou, S. J.; Qiu, J. T.; Gao, D. Y.; Lin, C. C. et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat. Nanotechnol.2019, 14, 1160–1169.
Kumari, R.; Sunil, D.; Ningthoujam, R. S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Control. Release2020, 319, 135–156.
Cai, L. H.; Hu, C. L.; Liu, S. N.; Zhou, Y.; Liu, Z. D.; Pang, M. L. Covalent organic framework-titanium oxide nanocomposite for enhanced sonodynamic therapy. Bioconjugate Chem.2021, 32, 661–666.
Wang, X.; Wang, W. P.; Yu, L. D.; Tang, Y.; Cao, J. Y.; Chen, Y. Site-specific sonocatalytic tumor suppression by chemically engineered single-crystalline mesoporous titanium dioxide sonosensitizers. J. Mater. Chem. B2017, 5, 4579–4586.
Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron-hole recombination time at TiO2 single-crystal surfaces: Influence of surface band bending. J. Phys. Chem. Lett.2014, 5, 1953–1957.
Sha, B. Y.; Gao, W.; Cui, X. Y.; Wang, L.; Xu, F. The potential health challenges of TiO2 nanomaterials. J. Appl. Toxicol.2015, 35, 1086–1101.
Cai, R.; Chen, C. Y. The crown and the scepter: Roles of the protein corona in nanomedicine. Adv. Mater.2019, 31, 1805740.
Shanei, A.; Shanei, M. M. Effect of gold nanoparticle size on acoustic cavitation using chemical dosimetry method. Ultrason. Sonochem.2017, 34, 45–50.
Garcia-Diaz, M.; Huang, Y. Y.; Hamblin, M. R. Use of fluorescent probes for ROS to tease apart Type I and Type II photochemical pathways in photodynamic therapy. Methods2016, 109, 158–166.
Dai, C.; Zhang, S. J.; Liu, Z.; Wu, R.; Chen, Y. Two-dimensional graphene augments nanosonosensitized sonocatalytic tumor eradication. ACS Nano2017, 11, 9467–9480.
Zhang, H. Y.; Pan, X. T.; Wu, Q. Y.; Guo, J.; Wang, C. H.; Liu, H. Y. Manganese carbonate nanoparticles-mediated mitochondrial dysfunction for enhanced sonodynamic therapy. Exploration2021, 7, 20210010.
Luo, Z. Y.; Tian, H.; Liu, L. L.; Chen, Z. K.; Liang, R. J.; Chen, Z.; Wu, Z. H.; Ma, A. Q.; Zheng, M. B.; Cai, L. T. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics2018, 8, 3584–3596.
Yang, B. W.; Chen, Y.; Shi, J. L. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev.2019, 119, 4881–4985.
Jiang, X. J.; Stockwell, B. R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol.2021, 33, 266–282.
Hassannia, B.; Vandenabeele, P.; Berghe, T. V. Targeting ferroptosis to iron out cancer. Cancer Cell2019, 35, 830–849.
Liang, C.; Zhang, X. L.; Yang, M. S.; Dong, X. C. Recent progress in ferroptosis inducers for cancer therapy. Adv. Mater.2019, 31, 1904197.
Gao, M. H.; Yi, J. M.; Zhu, J. J.; Minikes, A. M.; Monian, P.; Thompson, C. B.; Jiang, X. J. Role of mitochondria in ferroptosis. Mol. Cell2019, 73, 354–363.e3.
Li, Y. N.; Li, M. H.; Liu, L.; Xue, C. C.; Fei, Y.; Wang, X.; Zhang, Y. C.; Cai, K. Y.; Zhao, Y. L.; Luo, Z. Cell-specific metabolic reprogramming of tumors for bioactivatable ferroptosis therapy. ACS Nano2022, 16, 3965–3984.
Zhou, L. L.; Guan, Q.; Li, W. Y.; Zhang, Z. Y.; Li, Y. A.; Dong, Y. B. A ferrocene-functionalized covalent organic framework for enhancing chemodynamic therapy via redox dyshomeostasis. Small2021, 17, 2101368.
Efimova, I.; Catanzaro, E.; van der Meeren, L.; Turubanova, V. D.; Hammad, H.; Mishchenko, T. A.; Vedunova, M. V.; Fimognari, C.; Bachert, C.; Coppieters, F. et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer2020, 8, e001369.
Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G. M.; Apetoh, L.; Perfettini, J. L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med.2007, 13, 54–61.
Yang, W. S.; SriRamaratnam, R.; Welsch, M. E.; Shimada, K.; Skouta, R.; Viswanathan, V. S.; Cheah, J. H.; Clemons, P. A.; Shamji, A. F.; Clish, C. B. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell2014, 156, 317–331.
Zheng, Y. H.; Han, Y. B.; Sun, Q.; Li, Z. Harnessing anti-tumor and tumor-tropism functions of macrophages via nanotechnology for tumor immunotherapy. Exploration2022, 2, 20210166.
Carney, A. Y. Hyperbaric oxygen therapy: An introduction. Crit. Care Nurs. Quart.2013, 36, 274–279.
Castro, C. I.; Briceno, J. C. Perfluorocarbon-based oxygen carriers: Review of products and trials. Artif. Organs2010, 34, 622–634.
Tian, L.; Goldstein, A.; Wang, H.; Lo, H. C.; Kim, I. S.; Welte, T.; Sheng, K. W.; Dobrolecki, L. E.; Zhang, X. M.; Putluri, N. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature2017, 544, 250–254.
Hacker, L.; Brunker, J.; Smith, E. S.; Quirós-Gonzalez, I.; Bohndiek, S. E. Photoacoustics resolves species-specific differences in hemoglobin concentration and oxygenation. J. Biomed. Opt.2020, 25, 095002.
Kunst, R. F.; Verkade, H. J.; Oude Elferink, R. P. J.; van de Graaf, S. F. J. Targeting the four pillars of enterohepatic bile salt cycling; Lessons from genetics and pharmacology. Hepatology2021, 73, 2577–2585.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. GK202105004).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Sun, Y., Wang, Y., Han, R. et al. Ultrasound cascade regulation of nano-oxygen hybrids triggering ferroptosis augmented sonodynamic anticancer therapy. Nano Res. 16, 7280–7292 (2023). https://doi.org/10.1007/s12274-023-5377-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5377-0