Abstract
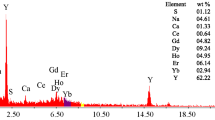
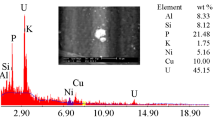
ataclastic rocks of Abu Rusheid area, Southeastern Desert of Egypt, were physically upgraded to obtain with producing a concentrate assaying 29.4% ZrO2, 1.55% Nb2O5, 3.13% RE2O3, 0.9% U3O8, and 0.66% ThO2. The corresponding minerals of these elements in the sample studied include zircon, columbite, samarskite, and uranothorite. Cassiterite is considered as an accessory mineral. The novelty of the suggested procedure is based on selective separation of niobium first from the aqueous liquor of the hydrous oxide cake obtained after alkali breakdown of the concentrate, leaving U, Th, Zr, and REEs in the residue. About 97% of niobium was selectively dissolved under the optimum conditions: weight ratio of concentrate sample to NaOH/KOH mixture 1 : 1.5, fusion time 2 h, and fusion temperature 500°C. This was followed by the recovery of Th, Zr, U, and REEs from the residue. The residue was treated with hydrochloric acid to achieve 98% leaching efficiency. From the two adequately obtained leach liquors, significant products have been prepared.








Similar content being viewed by others
REFERENCES
Ibrahim, M.E., Saleh, G.M., Ibrahim, I.H., Azab, M.S., Khamies, A.A., Oraby, F., Abu El-Hassan E.A., and Ragab, A.A., Geologic and ground spectrometric prospecting of the Abu Rusheid–Sikeit shear zones, South Eastern Desert, Egypt, Seventh Arab Conf. on the Peaceful Uses of Atomic Energy, Sanaa, 2004.
El-Afandy, A.H., El-Feky Mohamed, G., Taha, S., El Minyawi, S.M., and Sallam, H.A., Greener J. Geol. Earth Sci., 2016, vol. 4, no. 3, pp. 056–069. https://doi.org/10.15580/GJGES.2016.3.120916213
Mansour, G.R., Geological and geophysical investigations of mineralized structures in Wadi Abalea and its surroundings, Abu Rusheid area, South Eastern Desert, Egypt, PhD Thesis, Mansoura Univ., Egypt, 2005, p. 139
Huang, H., Ding, S., Su, D., Liu, N., Wang, J., and Tan, M., Sep. Purif. Technol., 2014, vol. 138, pp. 65–70. https://doi.org/10.1016/j.seppur.2014.10.008
Mastren, T., Radchenko, V., Owens, A., Cop**, R., Boll, R., Griswold, J.R., Mirzadeh, S., Wyant, L.E., Brugh, M., Engle, J.W., Nortier, F.M., Birnbaum, E.R., John, K.D., and Fassbender, M.E., Sci. Rep., 2017, vol. 7, ID 8216. https://doi.org/10.1038/s41598-017-08506-9
Mastren, T., Radchenko, V., Engle, J.W., Weidner, J.W., Owens, A., Wyant, L.E., Cop**, R., Brugh, M., Nortier, F.M., Birnbaum, E.R., John, K.D., and Fassbender, M.E., Anal. Chim. Acta, 2018, vol. 998, pp. 75–82. https://doi.org/10.1016/j.aca.2017.10.020
Afifi, S.Y., Saad, M.Z., Yousef, L.A., and Ismail, A.H., Arab J. Nucl. Sci. Appl., 2018, vol. 51, no. 2, pp. 10–19. http://doi.10.21608/AJNSA.2018.6506
Chung, K.W., Yoon, H-S., Kim, C.J., Lee, J.-Y., and Jyothi, R.K., J. Ind. Eng. Chem., 2020, vol. 83, pp. 72–80. https://doi.org/10.1016/j.jiec.2019.11.014
Kul, M., Topkaya, Y., and Karakaya, I., Hydrometallurgy, 2008, vol. 93, no. 3, p. 129. https://doi.org/10.1016/j.hydromet.2007.11.008
Hu, C., Liu, H.J., Peng, L., Sun, Y.K., and Long, W., J. Radioanal. Nucl. Chem., 2016, vol. 308, no. 1, p. 251. https://doi.org/10.1007/s10967-015-4306-z
Chellam, S. and Clifford, D.A., J. Environ. Eng., 2002, vol. 128, no. 10, pp. 942–952. https://doi.org/10.1061/(ASCE)0733-9372(2002)128:10(942)
Kuruc, J., Strisovska, J., Galanda, D., Dulanska, S., Matel, L., Jerigova, M., and Velic, D., J. Radioanal. Nucl. Chem., 2012, vol. 292, pp. 973–981. https://doi.org/10.1007/s10967-012-1670-9
Sharma, S. and Balasubramanian, K., RSC Adv., 2015, vol. 5, pp. 31732–31741. https://doi.org/10.1039/C5RA02861B
Stegnar, P. and Benedik, L., Arch. Oncol., 2001, vol. 9, pp. 251–255. https://inis.iaea.org/collection/NCLCollectionStore/_Public/34/047/34047643.pdf?r=1&r=1
The Health Hazards of Depleted Uranium Munitions, London: Royal Soc., 2001.
Li, P., Zhun, B., Wang, X., Liao, P., Wang, G., Wang, L., Guo, Y., and Zhang, W., Environ. Sci. Technol., 2017, vol. 51, no. 24, pp. 14368–14378. https://doi.org/10.1021/acs.est.7b05288
Yao, L., Min, X., Xu, H., Ke, Y., Liang, Y., and Yang, K., Int. J. Environ. Res. Public Health, 2018, vol. 15, no. 9. https://doi.org/10.3390/ijerph15091863
Yin, S., Wang, L., Kabwe, E., Chen, X., Yan, R., An, K., Zhang, L., and Wu, A., Minerals, 2018, vol. 8, no. 2, p. 32. https://doi.org/10.3390/min8020032
Quevauviller, Ph., Methodologies for Soil and Sediment Fractionation Studies, Cornwall, UK: Royal Soc. Chem., 2002.
Patra, A.C., Sumesh, C.G., Mohapatra, S., Sahoo, S.K., Tripathi, R.M., and Puranik, V.D., J. Environ. Manag., 2011, vol. 92, pp. 919–925. https://doi.org/10.1016/j.jenvman.2010.10.046
Zolfonoun, E., Monji, A.M., Taghizadeh, M., and Ahmadi, S.J., Miner. Eng., 2010, vol. 23, no. 9, pp. 755–756. http://doi.org/10.1016/j.mineng.2010.05.005
Abdel-Rehim, A.M., Int. J. Miner. Process., 2005, vol. 76, pp. 234–243. https://doi.org/10.1016/j.minpro.2005.02.004
Blumenthal, W.B., Zirconium and zirconium compounds, Kirk–Othmer Encyclopedia of Chemical Technology, Howe-Grant, M., Ed., New York: Wiley, 1998.
Manhique, R., Optimization of Alkali Fusion Process for Zircon Sands: A Kinetic Study of the Process, MSc Thesis, Faculty of Natural and Agricultural Sciences, Univ. of Pretoria, 2003.
Daher., A.M., Isot. Radiat. Res., 2008, vol. 40, no. 1, pp. 91–105.
Kwela, Z.N., Alkali-Fusion Processes for the Recovery of Zirconia and Zirconium Chemicals from Zircon Sand, MSc Thesis, Faculty of Natural and Agricultural Sciences, Univ. of Pretoria, 2000.
Deblonde, G.J.-P., Weigel, V., Bellier, Q., Houdard, R., Delvallée, F., Bélair, S., and Beltrami, D., Sep. Purif. Technol., 2016, vol. 162, pp. 180–187. https://doi.org/10.1016/j.seppur.2016.02.025
Rodriguez, M.H., Rosales, G.D., Pinna, E.G., and Suarez, D.S., Hydrometallurgy, 2016, vol. 159, pp. 60–64. https://doi.org/10.1016/j.hydromet.2015.10.033
El-Hussaini, O.M., Miner. Process. Extr. Metall. Rev., 2001, vol. 22, pp. 633–650. https://doi.org/10.1080/08827509808962519
Rodriguez, M.H., Rosales, G.D., Pinna, E.G., and Suarez, D.S., Hydrometallurgy, 2015, vol. 156, pp. 17–20. https://doi.org/10.1016/j.hydromet.2015.05.006
Deblonde, G.J.-P., Bengio, D., Beltrami, D., Belair, S., Cote, G., and Chagnes, A., Sep. Purif. Technol., 2019, vol. 215, pp. 634–643. https://doi.org/10.1016/j.seppur.2019.01.052
Deblonde, G.J.-P., Bengio, D., Beltrami, D., Belair, S., Cote, G., and Chagnes, A., Sep. Purif. Technol., 2019, vol. 226, pp. 209–217. https://doi.org/10.1016/j.seppur.2019.05.087
Abdel Wahab, G.M., Abdellah, W.M., Yousif, A.M., and Mubark, A.E., Min., Metall. Explor., 2022, vol. 39, pp. 833–846. https://doi.org/10.1007/s42461-019-00136-1
Wang, X., Zheng S., Xu, H., and Zhang, Y., Hydrometallurgy, 2009, vol. 98, pp. 219–223. https://doi.org/10.1016/j.hydromet.2009.05.002
Purcell, W., Potgieter, H., Nete, M., and Mnculwane, H., Min. Eng., 2018, vol. 119, pp. 57–66. https://doi.org/10.1016/j.mineng.2018.01.031
Amer, T.E., El-Assay, I.E., Rezk, A.A., El-Kammar, A.A., El-Manawy, A.W., and Abu Khoziem, H.A., Int. J. Min. Process., 2014, vol. 129, pp. 12–21. https://doi.org/10.1016/j.minpro.2014.04.005
Mubarak, A.E., Chemical Processing of Some Valuable Elements from Gabal El-Faliq Mineralizations, South Eastern Desert, Egypt, PhD Thesis, Faculty of Science, Menoufia Univ., 2019.
Krishnamurthy, N. and Gupta, C.K., Extractive Metallurgy of Rare Earths, CRC, 2004.
Horlait, D., Clavier, N., Szenknect, S., Dacheux, N., and Dubois, V., Inorg. Chem., 2012, vol. 51, pp. 3868–3878. https://doi.org/10.1021/ic300071c
Mathew, K.J., Burger, S., Ogt, S.V., Mason, P.M., and Narayanan, U.I., Uranium assay determination using Davies and Gray titration, Proc. Eighth Int. Conf. on Methods and Applications of Radioanalytical Chemistry (MARC VIII), Kaailua-Kona, Hawaii, 2009.
Marczenko, Z., Spectrophotometric Determination of Elements, Harwood, New York: Wiley, 2000.
Abdelkader, A.M., Daher, A., and El-Kashef, E., J. Alloys Compd., 2007, vol. 460, pp. 577–580. https://doi.org/10.1016/j.jallcom.2007.06.032
El-Nadi, Y.A., Daoud, J.A., and Aly, H.F., Int. J. Miner. Process., 2005, vol. 76, pp. 101–110. https://doi.org/10.1016/j.minpro.2004.12.005
Wang, X., Zheng, S., Xu, H., and Zhang, Y., Hydrometallurgy, 2009, vol. 98, pp. 219–223. https://doi.org/10.1016/j.hydromet.2009.05.002
Sharma, B.K., Nuclear Reaction Chemistry Book, GOEL, 2011, 7th ed., p. 348
Amer, T.E., El-Sheikh, E.M., Gado, M.A., Abu-Khoziem H.A., and Zaki, S.A., Sep. Sci. Technol., 2017, vol. 53, no. 10, pp. 1522–1530. https://doi.org/10.1080/01496395.2017.1405039
Kobayashi, T., Sasaki, T., Takagi, I., and Moriyama, H., J. Nucl. Sci. Technol., 2009, vol. 46, no. 11, pp. 1085–1090. https://doi.org/10.1080/18811248.2009.9711619
Crouthamel, C.E. and Martin, D.S.Jr., Ames Laboratory, 1950. http://lib.dr.iastate.edu/ameslab_iscreports/3
Habashi, F., A Textbook of Hydrometallurgy, Quebec, Canada, 1993.
El-Nadi, Y.A. and Daoud, J.A., J. Nucl. Radiochem. Sci., 2004, vol. 5, no. 1, pp. 11–15. https://doi.org/10.14494/jnrs2000.5.11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
El-Afandy, A.H., Yousif, A.M. & Mubark, A.E. Subsequent Separation of Niobium (Nb), Thorium (Th), Rare Earth Elements (REEs), Zirconium (Zr), and Uranium (U) from Abu Rusheid Cataclastic Concentrate, South Eastern Desert, Egypt. Radiochemistry 64, 257–267 (2022). https://doi.org/10.1134/S1066362222020175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222020175