Abstract
Obsessive–compulsive disorder (OCD) is associated with alterations in cortico-striato-thalamo-cortical brain networks, but some resting-state functional magnetic resonance imaging studies report more diffuse alterations in brain connectivity. Few studies have assessed functional connectivity within or between networks across the whole brain in unmedicated OCD patients or how patterns of connectivity predict response to exposure and ritual prevention (EX/RP) therapy, a first-line treatment for OCD. Herein, multiband resting-state functional MRI scans were collected from unmedicated, adult patients with OCD (n = 41) and healthy participants (n = 36); OCD patients were then offered twice weekly EX/RP (17 sessions). A whole-brain-network-based statistic approach was used to identify group differences in resting-state connectivity. We detected altered pre-treatment functional connectivity between task-positive regions in the temporal gyri (middle and superior) and regions of the cingulo-opercular and default networks in individuals with OCD. Signal extraction was performed using a reconstruction independent components analysis and isolated two independent subcomponents (IC1 and IC2) within this altered connectivity. In the OCD group, linear mixed-effects models tested whether IC1 or IC2 values predicted the slope of change in Yale–Brown Obsessive–Compulsive Scale (Y-BOCS) scores across EX/RP treatment. Lower (more different from controls) IC2 score significantly predicted greater symptom reduction with EX/RP (Bonferroni-corrected p = 0.002). Collectively, these findings suggest that an altered balance between task-positive and task-negative regions centered around temporal gyri may contribute to difficulty controlling intrusive thoughts or urges to perform ritualistic behaviors.
Similar content being viewed by others
Introduction
Obsessions and compulsions are the hallmarks of obsessive–compulsive disorder (OCD) and are thought to result from disruption of cortico-striato-thalamo-cortical (CSTC) feedback loops, specifically involving the anterior cingulate cortex, orbitofrontal cortex, and other frontoparietal regions [1,2,3,4,5,6,7]. The CSTC model has been investigated using resting-state functional magnetic resonance imaging (rsfMRI), which delineates brain connection-level or network-level functioning independent from a specific task. Most rsfMRI studies of OCD have focused on a priori subcortical (striatal) seed regions of interest (ROIs) and report alterations in frontal-subcortical connectivity in adults with OCD, consistent with the CSTC model [8,9,10,11]. These studies, however, differ in their specific findings (i.e., increased versus decreased connectivity in OCD) and many included medicated participants. Few studies have assessed functional connectivity within or between networks across the whole brain in unmedicated adults with OCD or examined how patterns of connectivity predict response to exposure and ritual prevention therapy (EX/RP), a first-line treatment for OCD.
Neuroimaging studies have begun examining disruptions extending beyond traditional CSTC loops in OCD [12,13,14,15,16]. Whole-brain studies of rsfMRI connectivity across large-scale intrinsic brain networks in unmedicated OCD participants have revealed broader alterations across task-positive and task-negative networks [17, 18]. “Task-positive” networks, including the frontoparietal (FPN), cingulo-opercular (CO), and ventral attention (VAN) networks, are typically engaged during goal-directed tasks requiring cognitive control and attention [19,20,21]. Of these task-positive networks, the CO functions to arbitrate how attention is divided among tasks and is alternatively referred to as a “salience” network [22]. The default-mode network (DMN) is a “task-negative” network, typically engaged during rest, self-referential processes, and mind wandering [23,24,25,26,27,28,29]. Findings from rsfMRI studies using seeds in or analyses restricted to the DMN, FPN, and salience/CO also suggest abnormal connectivity in OCD within and among these networks [30, 31]. Notably, previous whole-brain studies of adults [17] and children [18] reported decreased connectivity between and within these networks in OCD, while studies that focused exclusively on these networks [30, 31] reported increased connectivity across them. Also notable is that these prior studies included small samples ranging from 17 [17] to 35 [30] participants with OCD. Such findings suggest that OCD pathology may involve an impaired functional balance among task-positive and task-negative networks. For example, pediatric OCD findings from our lab [18] and meta-analytic findings from adults [6] suggest that the “triple network” model of psychopathology might apply to OCD, wherein the salience/CO might inappropriately “switch” or modulate between task-positive networks and the task-negative DMN [6, 18].
Probing whole-brain rsfMRI connectivity in adults may thus expand our understanding of the neurobiology of OCD beyond the CSTC model. However, the different methods used across previous studies complicate drawing conclusions about functional abnormalities in OCD, in part because findings from studies that analyze different brain regions are difficult to reconcile. For example, a study that does not include a particular ROI in analyses cannot be compared to studies with findings in that ROI. While a priori ROI selection can provide evidence for specific hypotheses such as the CSTC model, whole-brain approaches with good statistical power are necessary to search for more broad neural dysfunction across the brain.
RsfMRI studies have also begun to examine neural predictors of OCD treatment response. Cognitive-behavioral therapy (CBT) consisting of EX/RP is an evidence-based treatment for OCD. Although EX/RP has proven highly effective in randomized controlled trials, individual patients range in the degree of their response [32, 33]. Given the substantial investment of time and cost associated with EX/RP, the ability to predict which patients are likely to respond best could substantially improve clinical efficiency [34]. Prior rsfMRI findings suggest that EX/RP response can be predicted by changes in multiple brain areas, including the ventromedial prefrontal cortex [35] and DMN regions [36]. However, these studies included medicated patients despite data indicating that medication may affect neural activity in OCD [37]. Moreover, both studies focused on specific brain regions or networks defined a priori based on their established association with OCD, thereby precluding detection of abnormalities that do not overlap with regions identified in previous work. Two additional studies used whole-brain graph theoretical approaches to address the latter problem, but both used relatively small sample sizes [38, 39] (both n = 17) and the former also included medicated patients. As with the rsfMRI literature regarding neural changes in OCD, the heterogeneity in methods employed and in the OCD samples studied limits the ability to draw conclusions across studies about EX/RP response prediction.
To address this gap in the literature, we used a whole-brain approach in a larger sample of unmedicated patients to investigate patterns of functional connectivity that differ between adults with OCD and healthy controls (HC) and also predict EX/RP response in OCD patients. We used state-of-the-art multiband MRI acquisition sequences, taking advantage of recent advances in spatial and temporal resolution, and a whole-brain network-based statistic (NBS) technique to identify abnormalities in resting-state functional connectivity (rs-FC) in unmedicated adults with OCD. We then used these group differences to predict response to EX/RP in the OCD group. Based on previous findings from unmedicated participants with OCD, we hypothesized that the functional balance across task-positive and task-negative regions would be altered in OCD, and that such alterations would predict EX/RP response.
Methods and materials
Participants
Details of participant recruitment and data collection are published elsewhere [40]. Briefly, unmedicated adults with OCD and age-, sex-, and race/ethnicity-matched HC were recruited from the New York City area. All patients met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [41] criteria for OCD as confirmed by trained raters using the Structured Clinical Interview for Diagnostic and Statistical Manual-IV-TR Axis I Disorders [42]. Healthy participants had no lifetime psychiatric disorders. Participants were excluded for MRI contraindications, history of neurological illness or seizures, head trauma with loss of consciousness, pervasive developmental disorder, any current psychiatric diagnosis (other than OCD or phobias [if secondary] for the OCD group), or an estimated IQ < 80. The study was approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI). All participants provided written informed consent.
Treatment
Patients received a standardized protocol of EX/RP [43] that included 17 sessions (2 introductory and 15 exposure sessions, each 90-min long) delivered twice weekly by a clinical psychologist. Exposure sessions consisted of therapist-aided exposures, ritual prevention, and education about relapse prevention.
Clinical measures
Severity of OCD symptoms was assessed pre-, mid-, and post-treatment by a trained rater (independent from the treating clinician) using the Yale–Brown Obsessive Compulsive Scale (Y-BOCS) [44].
MRI acquisition
High-resolution MRI scans were conducted at NYSPI using sequences adapted from the Human Connectome Project (HCP) [45] for a GE Signa 3T MR750 scanner (details in Supplement). Briefly, each participant completed T1- and T2-weighted structural images and 2 runs of multiband resting-state functional MRI (repetition time = 850 ms, multiband factor = 6, 2 mm isotropic voxels = 7 min and 32 s per run). For the resting-state scans, participants were instructed to relax with their eyes open and remain awake.
MRI pre- and post-processing
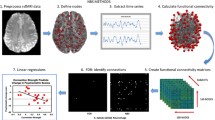
Functional data were preprocessed using the HCP pipelines v3.4 [46] (details in Supplement and Fig. S1). First-level analyses were performed in “grayordinate” space (“91k” CIFTI format), which combines cortical surface and subcortical volume representations. Post-processing was performed in Matlab R2019a (Mathworks, 2019). Nuisance regression was performed with 24 motion parameters (bandpass filtered [47]) and four average global grayordinate signal parameters, including squared terms, temporal derivatives, and squared temporal derivatives for all motion and global mean regressors [48]. Motion censoring [49, 50] based on framewise displacement (FD; >0.25 mm) and DVARS (z-score > 3) was also performed. All participants had at least 495 frames (7 min) of data remaining after censoring.
The brain was divided into 352 cortical and subcortical regions using a publicly available CIFTI-space segmentation (https://balsa.wustl.edu/file/show/JX5V) using HCP Connectome Workbench [51]. This segmentation included 333 cortical parcels from the Cortical Area Parcellation atlas defined by homogeneity of rs-FC [52] and 19 anatomically defined subcortical ROIs from the Freesurfer Subcortical Atlas: left and right amygdala, hippocampus, accumbens, caudate, pallidum, putamen, thalamus, ventral diencephalon, cerebellum, and brain stem. Next, the BOLD timeseries from each of the 352 regions/parcels (“nodes”) was extracted and correlated with all other nodes. These correlations (“edges”) were Fisher r-to-z transformed, resulting in a 352 × 352 connectivity matrix for each participant.
A growing consensus suggests that whole-brain analyses are sensitive to how the brain is parcellated and the granularity of these parcels [53, 54]. To test robustness of our NBS analyses (described below), we conducted a sensitivity analysis aimed to replicate findings using a coarser 100-parcel segmentation [55] (Supplement). In addition to having a substantially different number and granularity of ROIs, this parcellation was created using a different reference dataset, modeled using an algorithm accounting for both homogeneity and local gradient changes, and trained on both task and resting-state fMRI data.
Statistical analysis
Participant characteristics
Group differences in participant characteristics were tested using t-tests for continuous variables and χ2 tests for categorical variables in Matlab.
Group differences in functional connectivity
Whole-brain correlation matrices were examined for group differences between OCD patients and controls using the Network Based Statistic (NBS Connectome v1.2 [56]). NBS uses permutation testing to identify significant components or “clusters” of contiguous region-to-region connections and to correct for multiple comparisons (10,000 permutations), which can control the family-wise error rate with more power than mass univariate testing in cases where the brain connectivity features of interest are interconnected [56] (see Supplement).
Group differences were tested using an F-test to examine both OCD > HC and OCD < HC differences parsimoniously in one test (rather than two unidirectional t-tests), controlling for age, sex, and mean FD to control for residual effects of head motion during the MRI scan. Extent thresholding and an f-statistic threshold of 14 (p = 0.0004) were used for primary analyses. Findings from NBS with extent and intensity thresholding across a range of f-statistic thresholds are reported in the supplement. Significant components were visualized using the BrainNet Viewer [Strengths Our study had several strengths. First, we used multiband acquisition sequences and state-of-the-art image processing pipelines (e.g., the HCP pipeline [46]). Second, to address concerns of the impact of head motion on rs-FC data, we conducted motion denoising via nuisance regression with squared terms, temporal derivatives, and squared temporal derivatives for each of six realignment parameters (translation and rotation in each of the x, y, and z planes) based on a recent benchmarking study showing that this 24-parameter method outperformed alternatives with fewer motion-related regressors [48]. We additionally performed motion censoring [49] using relatively strict cutoffs based on FD and DVARS and included FD as a covariate in analyses to further reduce the residual effects of head motion on results. Third, we reproduced our analyses of group differences using two contrasting parcellations, one finer [52] and one coarser [55], suggesting that our results are robust and not an artifact of parcellation scheme. Finally, we enrolled only unmedicated patients with minimal comorbidities, and 34 out of 41 patients (83%) were drug-naïve, minimizing the confound of medication use on our brain findings. Our sample, although comparable to or larger than previous rs-FC studies of unmedicated adults with OCD, was still relatively small and 73% non-Hispanic white. In addition, our study design compared OCD patients undergoing EX/RP therapy with HC participants, but did not have a positive control arm (e.g., an OCD group undergoing placebo or an alternative treatment). Future work should explore neural predictors of treatment response in a large, randomized control trial with EX/RP, medication, and placebo arms to examine specificity. Finally, our study was not powered to test treatment response prediction in an independent validation sample [83], but does set the stage for future studies to examine whether baseline rs-FC centered around the MTG/STG predicts EX/RP response in an independent sample. Comparing findings across neuroimaging studies are difficult due, in part, to differences in methods. To address this challenge, we recommend future rs-FC studies in OCD standardize preprocessing to help with transparency and replication [84] and use whole-brain approaches to expand our understanding of OCD beyond existing models. In addition, we recommend that researchers report ROIs and findings by coordinates rather than just by name or description, make imaging (e.g., NIFTI or CIFTI format) files containing masks of these regions freely available online, and highlight in manuscripts when a network being studied has multiple names. Sensitivity analyses can be used to test if findings replicate across different atlases, as choice of parcellation can influence functional connectivity-based results [53, 54]. Finally, future studies that assess the comparability of multiband rs-FC measurements to those from traditional singleband sequences would be helpful in understanding how newer imaging results compare to prior findings.Limitations
Future directions
Conclusions
Our study is the first to combine multiband rs-FC with a whole-brain network-based approach to assess rs-FC abnormalities in unmedicated adults with OCD and rs-FC predictors of response to EX/RP. Functional connectivity in a component dominated by edges connecting task-positive temporal gyri regions to other task-positive (salience/CO) and task-negative (DMN) regions was altered in patients with OCD. Furthermore, those patients with lower functional connectivity across these regions showed better response to EX/RP. These findings suggest that altered connectivity across task-positive and task-negative temporal gyri regions may be central to OCD pathophysiology. Since rs-FC is relatively easy to acquire consistently across sites and scanners, this work highlights the need for future rs-FC studies of EX/RP response in unmedicated OCD patients to validate the predictive value of these findings in an independent sample.
Funding and disclosure
This work was supported by grants from the National Institute of Mental Health (R01MH104648; Principal Investigators: RM and HBS) and the National Institutes of Health (MSTP Training Grant to Columbia University T32GM007367, supporting TCS). In the last 3 years, HBS has received research funds from Biohaven (2018 to present), royalties from Cambridge University Press and UpToDate, Inc., and a stipend for serving as associate editor of JAMA Psychiatry (2019 to present). The remaining authors declare no competing interests.
References
Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20:1251–83.
Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–23.
Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–74.
Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron 2000;28:343–7.
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Gursel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018;87:151–60.
Marsh R, Horga G, Parashar N, Wang Z, Peterson BS, Simpson HB. Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry. 2014;75:615–22.
Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–200.
Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2852–60.
Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishimura T, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry. 2011;26:463–9.
Zhao Q, Xu T, Wang Y, Chen D, Liu Q, Yang Z, et al. Limbic cortico-striato-thalamo-cortical functional connectivity in drug-naive patients of obsessive-compulsive disorder. Psychol Med. 2019:1–13.
Moody TD, Morfini F, Cheng G, Sheen C, Tadayonnejad R, Reggente N, et al. Mechanisms of cognitive-behavioral therapy for obsessive-compulsive disorder involve robust and extensive increases in brain network connectivity. Transl Psychiatry. 2017;7:e1230.
Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51.
Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, et al. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Hum Brain Mapp. 2012;33:1089–106.
Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73:815–25.
Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, et al. Error processing and inhibitory control in obsessive-compulsive disorder: a meta-analysis using statistical parametric maps. Biol Psychiatry. 2019;85:713–25.
Gottlich M, Kramer UM, Kordon A, Hohagen F, Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:5617–32.
Cyr M, Pagliaccio D, Yanes-Lukin P, Fontaine M, Rynn MA, Marsh R. Altered network connectivity predicts response to cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Neuropsychopharmacology. 2020,45:1232–40.
Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6.
Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–43.
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7.
Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8.
Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13.
Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–70.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, et al. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci U S A. 2008;105:9781–6.
Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science 2007;315:393–5.
Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, et al. Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J Affect Disord. 2017;223:106–14.
Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, et al. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2017;38:678–87.
Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151–61.
Simpson HB, Huppert JD, Petkova E, Foa EB, Liebowitz MR. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. 2006;67:269–76.
O’Neill J, Feusner JD. Cognitive-behavioral therapy for obsessive-compulsive disorder: access to treatment, prediction of long-term outcome with neuroimaging. Psychol Res Behav Manag. 2015;8:211–23.
Fullana MA, Zhu X, Alonso P, Cardoner N, Real E, Lopez-Sola C, et al. Basolateral amygdala-ventromedial prefrontal cortex connectivity predicts cognitive behavioural therapy outcome in adults with obsessive-compulsive disorder. J Psychiatry Neurosci. 2017;42:378–85.
Reggente N, Moody TD, Morfini F, Sheen C, Rissman J, O’Neill J, et al. Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive-compulsive disorder. Proc Natl Acad Sci USA. 2018;115:2222–27.
Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–29.
Gottlich M, Kramer UM, Kordon A, Hohagen F, Zurowski B. Resting-state connectivity of the amygdala predicts response to cognitive behavioral therapy in obsessive compulsive disorder. Biol Psychol. 2015;111:100–9.
Feusner JD, Moody T, Lai TM, Sheen C, Khalsa S, Brown J, et al. Brain connectivity and prediction of relapse after cognitive-behavioral therapy in obsessive-compulsive disorder. Front Psychiatry. 2015;6:74.
Pagliaccio D, Middleton R, Hezel D, Steinman S, Snorrason I, Gershkovich M, et al. Task-based fMRI predicts response and remission to exposure therapy in obsessive-compulsive disorder. Proc Natl Acad Sci USA. 2019;116:20346–53.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub: Philadelphia; 2013.
First MB, Spitzer RL, Gibbon M, Williams JB. SCID-I/P. Biometrics Research, New York State Psychiatric Institute: New York; 2002.
Foa EB, Yadin E, Lichner TK. Exposure and response (ritual) prevention for obsessive compulsive disorder: therapist guide. Oxford University Press: New York; 2012.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11.
Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, et al. The Human Connectome Project: a data acquisition perspective. Neuroimage 2012;62:2222–31.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 2013;80:105–24.
Fair DA, Miranda-Dominguez O, Snyder AZ, Perrone A, Earl EA, Van AN, et al. Correction of respiratory artifacts in MRI head motion estimates. Neuroimage 2020;208:116400.
Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 2017;154:174–87.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–54.
Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 2015;105:536–51.
Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, et al. Informatics and data mining tools and strategies for the human connectome project. Front Neuroinform. 2011;5:4.
Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26:288–303.
Messe A. Parcellation influence on the connectivity-based structure-function relationship in the human brain. Hum Brain Mapp. 2020;41:1167–80.
Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C, et al. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage 2010;50:970–83.
Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095–114.
Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage 2010;53:1197–207.
**a M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910.
Le QV, Karpenko A, Ngiam J, Ng AY. ICA with reconstruction cost for efficient overcomplete feature learning. Proceedings of the 24th International Conference on Neural Information Processing Systems. 2011:1017–25.
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, et al. Package ‘lme4’. Convergence. 2015;12:2.
Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014;205:376–82.
Chen Y, Meng X, Hu Q, Cui H, Ding Y, Kang L, et al. Altered resting-state functional organization within the central executive network in obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2016;70:448–56.
Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605.
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS ONE. 2012;7:e36356.
Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78.
Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31.
Hou JM, Zhao M, Zhang W, Song LH, Wu WJ, Wang J, et al. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. J Psychiatry Neurosci. 2014;39:304–11.
Jang JH, Kim JH, Jung WH, Choi JS, Jung MH, Lee JM, et al. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–62.
Sha Z, Edmiston EK, Versace A, Fournier JC, Graur S, Greenberg T, et al. Functional disruption of cerebello-thalamo-cortical networks in obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:438–47.
Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science 2010;329:1358–61.
Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2014;44:1121–30.
Kalanthroff E, Henik A, Simpson HB, Todder D, Anholt GE. To do or not to do? Task control deficit in obsessive-compulsive disorder. Behav Ther. 2017;48:603–13.
Jung J, Cloutman LL, Binney RJ, Lambon, Ralph MA. The structural connectivity of higher order association cortices reflects human functional brain networks. Cortex 2017;97:221–39.
Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, et al. Spontaneous neural activity in the right superior temporal gyrus and left middle temporal gyrus is associated with insight level in obsessive-compulsive disorder. J Affect Disord. 2017;207:203–11.
Kim M, Kwak S, Yoon YB, Kwak YB, Kim T, Cho KIK, et al. Functional connectivity of the raphe nucleus as a predictor of the response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. Neuropsychopharmacology 2019;44:2073–81.
Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature 2016;536:171–78.
Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 2013;80:169–89.
McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry. 2011;16:592–4.
Simmons AN, Arce E, Lovero KL, Stein MB, Paulus MP. Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. Int J Neuropsychopharmacol. 2009;12:1009–20.
van den Heuvel OA, Boedhoe PSW, Bertolin S, Bruin WB, Francks C, Ivanov I, et al. An overview of the first 5 years of the ENIGMA obsessive-compulsive disorder working group: the power of worldwide collaboration. Hum Brain Mapp. 2020.
Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: a review. JAMA Psychiatry. 2020;77:534–40.
Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci. 2011;22:1359–66.
Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–42.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain map**. Hum Brain Mapp. 2000;10:120–31.
Acknowledgements
We acknowledge and thank Martine Fontaine and all members of the Center for OCD and Related Disorders (e.g., therapists, clinical raters, research assistants, data managers) who worked on this study as well as all research participants. This study has been registered as a clinical trial (Control and Reward Circuits in Obsessive Compulsive Disorder, NCT02221518); additional information is provided at https://clinicaltrials.gov/ct2/show/NCT02221518.
Author information
Authors and Affiliations
Contributions
RM and HBS designed the study; TCS, DP, and RM analyzed the data; and all authors contributed to writing and/or editing the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shi, T.C., Pagliaccio, D., Cyr, M. et al. Network-based functional connectivity predicts response to exposure therapy in unmedicated adults with obsessive–compulsive disorder. Neuropsychopharmacol. 46, 1035–1044 (2021). https://doi.org/10.1038/s41386-020-00929-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-020-00929-9
- Springer Nature Switzerland AG
This article is cited by
-
A clinical investigation of psychotic vulnerability in early-onset Obsessive–Compulsive Disorder through Cognitive–Perceptive basic symptoms
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Functional connectivity abnormalities of brain networks in obsessive–compulsive disorder: a systematic review
Current Psychology (2024)
-
Probing midbrain dopamine function in pediatric obsessive-compulsive disorder via neuromelanin-sensitive magnetic resonance imaging
Molecular Psychiatry (2023)