Highlights
-
3D cubic hollow core–shell NiCo2O4@C composites were synthesized.
-
Oxygen vacancies were introduced into the prepared composites.
-
Ultrabroad effective absorption bandwidth of 12.48 GHz was obtained.
-
The absorption performance mechanism of NiCo2O4/C composites was investigated.
Abstract
Cobalt nickel bimetallic oxides (NiCo2O4) have received numerous attentions in terms of their controllable morphology, high temperature, corrosion resistance and strong electromagnetic wave (EMW) absorption capability. However, broadening the absorption bandwidth is still a huge challenge for NiCo2O4-based absorbers. Herein, the unique NiCo2O4@C core–shell microcubes with hollow structures were fabricated via a facile sacrificial template strategy. The concentration of oxygen vacancies and morphologies of the three-dimensional (3D) cubic hollow core–shell NiCo2O4@C framework were effectively optimized by adjusting the calcination temperature. The specially designed 3D framework structure facilitated the multiple reflections of incident electromagnetic waves and provided rich interfaces between multiple components, generating significant interfacial polarization losses. Dipole polarizations induced by oxygen vacancies could further enhance the attenuation ability for the incident EM waves. The optimized NiCo2O4@C hollow microcubes exhibit superior EMW absorption capability with minimum RL (RLmin) of −84.45 dB at 8.4 GHz for the thickness of 3.0 mm. Moreover, ultrabroad effective absorption bandwidth (EAB) as large as 12.48 GHz (5.52–18 GHz) is obtained. This work is believed to illuminate the path to synthesis of high-performance cobalt nickel bimetallic oxides for EMW absorbers with excellent EMW absorption capability, especially in broadening effective absorption bandwidth.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The emergence of various wireless electronic devices was promoted by the rapid development of science and technology, provides people with convenience. However, the adverse effects of the operation and maintenance of sophisticated instruments, human organ damage, and even the survival of certain organisms were also produced by the electromagnetic radiation, which was called electromagnetic pollution [1,2,3]. For the sake of dealing with electromagnetic pollution, EMW absorbers have received numerous attentions due to their ability to weaken or/and dissipate EMW by converting them into other forms of energy [4,
The phase structure and crystallinity of precursors and NCO is characterized by XRD (Figs. 2a and S7). The Ni–Co PBA precursors in Fig. S1 is detected as Ni3[Co(CN)6]2·12H2O (JCPDS NO. 89–3738). Then the Ni–Co PBA precursors transform into NiCo2O4/C hybrids, where the obvious diffraction peaks located at the 2θ of 18.91°, 31.15°, 36.70°, 44.62°, 59.10° and 64.98° in Fig. 2a are attributed to the (111), (220), (311), (400), (511), and (440) planes of NiCo2O4 (PDF #NO.20–0781) [20]. These results further prove the successful preparation of NiCo2O4/C microcubes.
a XRD patterns, b Raman test of the prepared NCO; c Nitrogen adsorption/desorption isotherms and the pore size distributions, d XPS spectra of NCO-3, high-resolution XPS spectra for e C 1s, f) Co 2p and g) Ni 2p of NCO-3; XPS spectra of O 1s of h NCO-1, i NCO-2, j NCO-3 and k NCO-4; l the magnetization curve of NCO
Figure 2b shows the Raman spectra of the prepared composite, where the four distinct stretching vibration peaks at 184, 463, 510, and 651 cm−1 are detected as F2g, Eg, F2g, and A1g signals of NiCo2O4 orderly [21, 22], which further prove the formation of NiCo2O4. The peaks at ~ 510 and ~ 651 cm−1 are in connection with the vibration of oxygen ions (tetrahedral) and Co–O (octahedral), separately. The differences in F2g and A1g peak positions for the four samples, where 517 and 660 cm−1 for NCO-1, 509 and 656 cm−1 for NCO-2 and 509 and 651 cm−1 for NCO-4, is attribute to the disruption of lattice symmetry by defects caused during the calcination process. The peaks move towards lower frequencies with the increase of heat treatment temperature that are inferred to promote the generation of more oxygen vacancy defects, which induces defect polarization to enhance the electromagnetic absorption property [23]. To explore the detailed microstructure characteristics of the prepared composites, the N2 adsorption/desorption isotherms and pore size distribution curve of NCO-3 are displayed in Fig. 2c. The typical type IV isotherms can be observed directly, indicating the presence of mesoporous structure [24,25,26]. The specific surface area of NCO-3 is calculated to be ca. 11.2 m2 g−1, and the pore size mainly distributes between 1 and 150 nm.
The valence state and chemical composition of the prepared NiCo2O4/C hybrids is explored by the XPS test (Fig. 2d–k). The XPS spectra of NCO-3 illustrate the existing elements of C 1s, Co 2p, Ni 2p, and O 1s, which further confirm the successful synthesis of NiCo2O4/C composites. The three split peaks in the spectra of C 1s in Fig. 2e are attributed to the C–C (284.6 eV)、C–O (285.6 eV), and O–C=O (288.5 eV), respectively [27]. The Co 2p spectra (Fig. 2f) can be fitted to two spin–orbit doublets characteristics of Co2+ (780.98 and 796.5 eV) and Co3+ (788 and 805 eV), accompanying two satellite peaks (788 and 805 eV) [28, 29]. Meanwhile, the Ni 2p emission spectra (Fig. 2g) can be fitted to two spin–orbit doublets features of Ni2+ and Ni3+, accompanying two shakeup satellites [18]. Similarly, the spectra of Ni 2p are composed of two satellite peaks (862 and 880 eV) and two spin–orbit doublets features of Ni2+ (854 and 872 eV) and Ni3+ (856 and 874 eV) [30]. The O 1 s spectrum consists of four categories of oxygen species (Fig. 2h–k). The peaks at 529.5 and 531.2 eV accord to the Ni–O and Co–O bond, respectively [31]. Simultaneously, the peak at 530.7 eV is related to oxygen vacancy, illustrating the presence of oxygen vacancy defects, consistent with the electron paramagnetic resonance (EPR) test results (Fig. S8). In our previous study, it was been proved that the content of oxygen vacancy defects is positively correlated with the electromagnetic wave absorption performance [32]. Therefore, the relative oxygen vacancy content of the NiCo2O4/C hybrids was calculated and compared based on the proportion of the integral area in Table S1. The NCO-3 presents the highest content of oxygen vacancies, which is favorable to generate dipole polarizations, illustrating the content of oxygen vacancy defects increases by the heat treatment temperature, and also influenced by structural integrity of the composites. Besides, the peak in 532.5 eV is indexed to the adsorbed water on the material surface [33].
To further detect the existence of carbon matrix in the prepared composites, the TGA measurement of NCO-3 is displayed in Fig. S9. In the process of heating, the mass of the sample gradually decreases; the weight change may be caused by the evaporation of water, the combustion of carbon, the decomposition and oxidation of the –CN– bond that generates carbon and nitrogen oxide gas and escapes into the air, and the conversion of NiCo2O4 into NiO and Co3O4 after heating up to 500 °C. in which Co2+ is oxidized to Co3+, and Ni3+ and O2 molecules are reduced to Ni2+ and O2− [34].
The static magnetic property of the NiCo2O4 is tested at room temperature and shown in Fig. 2l. The coercively values of the absorbers are all around 251 Oe, indicating the similar storage capacity of magnetic energy in this series of samples [35]. The saturation magnetization (Ms) value gradually decreases with increasing temperature, and the maximum Ms was only 3.45 emu g.−1 for the NCO-1, which illustrate the hysteresis loss with low saturation magnetization contributes little to the magnetic loss of the absorbers. [36]
EPR results in Fig. S8 prove the existence of oxygen vacancy (OV) [20], which depends on Eq. (1) [24]:
where \(h\) represents Planck’s constant, \(v\) is frequency, \(g\) means constant, \(\beta\) means Bohr magneton, and \(B\) mean applied magnetic field. The recognized \(g\) value of materials with OV is ca. 2.00, which is associated with the nature of radical. The \(g\) value of the NiCo2O4/C hybrids is calculated to be 2.00477, 2.00466, 2.00497, and 2.00494, respectively, which further indicate the occurrence of oxygen vacancy. The oxygen vacancies act as point defects, which will trap electrons and disrupt the equilibrium distribution of charges, leading to polarization and loss of electromagnetic energy under the altering electric field.
The EMW absorbing properties of the synthesized NiCo2O4/C composites (with filling ratio of 40%) are dominated by the complex permittivity (\(\varepsilon_{r}\) = \(\varepsilon^{\prime }\) − \(j\varepsilon^{\prime \prime }\)) and permeability (\(\mu_{r}\) = \(\mu^{\prime }\) − \(j\mu^{\prime \prime }\)). The electromagnetic parameters of the absorbers are obtained from the vector network analyzer test that shown in Fig. 3. The real parts of permittivity (\(\varepsilon^{\prime }\)), decreases accompanied by the increasing frequency, and some fluctuations are observed in the curve (Fig. 3a). The imaginary part (\(\varepsilon^{\prime \prime }\)) of the complex permittivity (Fig. 3b) and dielectric loss tangent (Fig. 3c) presents multiple vibrational peaks from 6.5 to 18 GHz, indicating the existence of Debye relaxation, which corresponds well with the results of Cole–Cole semicircles (Fig. 3g–j). The dipole polarization formed in the prepared absorbers due to the abundant oxygen vacancies. And interfacial polarization between heterogeneous interfaces (NiCo2O4, carbon, and air) is inferred to contribute to satisfactory properties.
The complex permeability of the absorbers fluctuates in a low-value range (0.79 to 1.32 and −0.28 to 0.21, respectively) for both \(\mu^{\prime }\) and \(\mu^{\prime \prime }\) (Fig. 3d, e), which indicates a relatively poor magnetic loss capability [6]. The multiple resonance peaks (Fig. 3f) mainly originate from exchange resonance and natural resonance. Besides, eddy current loss is also an essential magnetic loss mechanism, which is described by the eddy current coefficient \((C_{0} = \mu^{\prime \prime } \left( {\mu^{\prime } } \right)^{2} f^{ - 1} )\) [37]. As shown in Fig. 3l, the \(C_{0}\) of the NiCo2O4/C absorbers is almost a constant in the range of 6 to 18 GHz, suggesting a nonnegligible role of eddy current loss in the magnetic loss behavior.
The RL values are calculated according to transmission line theory, as shown in Eqs. 2 and 3 [38]:
where Zin means the input impedance of the absorber, Z0 means the impedance of free space, \({\varepsilon }_{r}\) and \({\mu }_{r}\) represent the complex permittivity and complex permeability, respectively, \(f\) means the frequency of the incident electromagnetic wave, \(d\) is the thickness and c is the speed of the EMW. The RL value less than −10 dB illustrates more than ninety percent of the incident EMW could be absorbed and the corresponding frequency range with RL lower than −10 dB is considered as EAB [22].
The EMW absorption performance of the as-synthesized NiCo2O4@C microcubes is presented in Figs. 4 and 5. The results illustrate NiCo2O4@C microcubes present outstanding EMW absorption performance in terms of the calculated RL and EAB values. An EAB value of 11.44 GHz for NCO-1 is obtained at 3.0 mm and the RLmin value reaches −56.51 dB at the thickness of 2.5 mm (Figs. 4a, b and 5a). For NCO-2, the EAB value increases to 11.68 GHz at 3.0 mm, and the RLmin value of −78.92 dB is obtained at 2.0 mm (Figs. 4c, d and 5b). A satisfactory EAB value of 11.12 GHz obtained at 3.0 mm and RLmin value of −49.23 dB at 2.0 mm are obtained for NCO-4, respectively (Figs. 4g, h and 5d). Encouragingly, an ultra-large EAB value as broad as 12.48 GHz (5.52 to 18 GHz) and RLmin value of −84.45 dB at the thickness of 3.0 mm (Figs. 4e, f and 5c), which is the largest among the NiCo2O4-based absorbers (Table 1). It is worth noting that none RL value for EMW absorbers at multiple matched thicknesses beyond −10 dB at 18 GHz, indicating that the absorbers display potential absorption capability in the higher frequency range. The absorption performance of the four EMW absorbers is compared in more detail by reducing the matching thickness from 0.5 to 0.2 mm, as shown in Fig. 5e–l, where the EAB of all the four EMW absorbers exceed 10 GHz and RLmin values exceed −20 dB (losing 99% of the incident electromagnetic wave) at these matched thicknesses. Figure 5m, n shows the comparison of EMW Absorption performance for all absorbers at the thickness of 3 and 5 mm, respectively, which further prove the NCO-3 possessed the optimum EAB value. Meanwhile, the EAB value even reaches 14.96 GHz at the thickness of 5.0 mm.
RL values vs. frequency at different thicknesses, the simulations of the absorber thickness vs. peak frequency under l/4 conditions, and the impedance matching characteristic for a NCO-1, b NCO-2, c NCO-3, and d NCO-4. Comparison of e–h EAB values and i–l RLmin at a matched thickness of 3–5 mm. Comparison of EMW absorption performance at a thickness of m 3 mm and n 5 mm
In order to investigate the attenuation mechanism for the excellent EMW absorption, the attenuation constant (α) is evaluated using Eq. 4 [39]:
Figure 3k displays the α-values of the four prepared absorbers, which shows the result of NCO-4 > NCO-2 > NCO-1 > NCO-3. Theoretically, NCO-4 should present the optimum EMW absorption capacity, but the impedance matching factor should be considered.
For exploring deeper into the cause of the excellent EM wave dissipation behavior of the NiCo2O4/C absorbers, the impedance matching characteristic (\(Z= |{Z}_{in}/{Z}_{0}|\)) is computed and exhibited in Figs. 4 and 5. Typically, the better the impedance matching for the absorber [40], the less reflection the incident EMW. The results show that the impedance matching of the prepared absorbers floats around 1.0 at a suitable matching thickness [41]. By further comparative analysis, NCO-3 displays the most outstanding impedance match. Based on the synergistic effect of impedance matching and attenuation constants, the NCO-3 presents a superior EMW absorption capability.
Furthermore, EMW absorption capability of NCO-3 with a filling ratio of 20% and 30% was tested, which are presented in Figs. S10–S12. The NCO-3 with filling ratio of 20% exhibits an EAB of 5.44 GHz at 3.0 mm and an RLmin value of −20.44 dB at 3.5 mm and NCO-3 with filling ratio of 30% exhibits an EAB value of 4.96 GHz at 2.5 mm and an RLmin value of −46.02 dB at 5.0 mm, respectively, which demonstrates noteworthy impact of the filling ratio for EMW absorption capability.
For better understanding of the outstanding EMW absorption performance of the NiCo2O4/C absorber, the quarter wavelength theory was applied by Eq. 5 [42]:
where \(t_{m}\) and \(f_{m}\) mean the optimal thickness and frequency of the absorbers. The matching results of RLmin, thickness, and frequency are shown in Fig. 5. The intersection of the dashed line and the t-curve indicate the matching thickness vs. the peak frequency collected in Fig. 5a–d. The positions of these data are around the 1/4 λ, indicating these absorbers are in accordance with the quarter wavelength theory. Therefore, incident and reflected EMW would be canceled by each other in the interface of air-absorbers as the phase difference of 180°, maximizing the EMW attenuation ability.
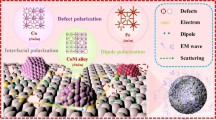
The outstanding EMW dissipation of NCO-3 with an ultrabroad EAB value of 12.48 GHz is achieved, and the attenuation mechanisms are summarized in Fig. 6. The outstanding impedance matching characteristics allow EMW to enter the absorbers to the greatest extent firstly. Secondly, these controllable oxygen vacancy defects act as dipoles and induce dipole polarization. The dipoles would change their arrangement from disorder to order when an electric field is applied, and the incident EMW will be effectively dissipated during this period [43]. Thirdly, the existence of eddy current loss and interface polarization induced by the accumulation of different charges at the heterogeneous interfaces, are beneficial to the loss effect of EMW. Fourthly, the different binding energy for electric charges on the sides of the interface leads to differences in the amount of charge migration in and out of electromagnetic fields, resulting in interface polarization. Finally, when the EMW enters the inside framework, multiple reflections between the gaps are happened to increase the propagation path and then dissipate the EM waves [44, 45]. Furthermore, macroscopic current would be generated in the absorber under an applied electromagnetic field, and the EMW would be converted into heat energy based on the Joule theory.
4 Conclusions
In summary, the 3D core–shell NiCo2O4@C hollow microcubes are synthesized by the environmentally-friendly sacrificial template method. The oxygen vacancy defects are introduced into the special framework due to the sensitive response of NiCo2O4 to temperature changes. The evolution of morphology with temperature affected the density, distribution of heterogeneous interfaces, and the content of oxygen vacancy defects of the prepared composites, thus affecting the impedance matching and attenuation capability of the absorber. Benefiting from the synergistic EMW absorption mechanisms, including multiple reflections and scattering induced by the abundant pores, interfacial polarization induced by multiple components, dipole polarization induced by the defects, conductive loss, and eddy current loss, the optimized NCO-3 presented ultrabroad EAB value of 12.48 GHz (5.52 to 18 GHz), and RLmin value of −84.45 dB at 8.4 GHz, covering the entire X and Ku band and even extends to the K band, which is the broadest among the reported NiCo2O4-based absorbers. It is believed that this report could illuminate the route for applications of high-performance NiCo2O4-based and other transition metal oxides-based EMW absorbers.