Abstract
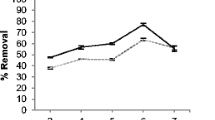
Biosorption is a promising technology for the removal of heavy metals from industrial wastes and effluents. In the present study, biosorption of Pb2+, Cu2+, Fe2+ and Zn2+ onto the dried biomass of Eucheuma denticulatum (Rhodophyte) was investigated as a function of solution pH, contact time, temperature and initial metal ion concentration. The experimental data were evaluated by Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherm models. The sorption isotherm data followed Langmuir and Freundlich models, and the maximum Langmuir monolayer biosorption capacity was found as 81.97, 66.23, 51.02 and 43.48 mg g−1 for Pb2+, Cu2+, Fe2+ and Zn2+, respectively. The sorption kinetic data followed pseudo-second-order and intraparticle diffusion models. Thermodynamic study revealed feasible, spontaneous and endothermic nature of the sorption process. Fourier transform infrared analysis showed the presence of amine, aliphatic, carboxylate, carboxyl, sulfonate and ether groups in the cell wall matrix involved in metal biosorption process. A total of nine error functions were applied in order to evaluate the best-fitting models. We strongly suggest the analysis of error functions for evaluating the fitness of the isotherm and kinetic models. The present work shows that E. denticulatum can be a promising low-cost biosorbent for removal of the experimental heavy metals from aqueous solutions. Further study is warranted to evaluate its potential for the removal of heavy metals from the real environment.





Similar content being viewed by others
References
Al-Homaidan AA, Al-Houri HJ, Al-Hazzani AA, Elgaaly G, Moubayed NMS (2014) Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arabian J Chem 7:57–62. doi:10.1016/j.arabjc.2013.05.022
Arshadi M, Amiri MJ, Mousavi S (2014) Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour Ind 6:1–17. doi:10.1016/j.wri.2014.06.001
Basha S, Murthy ZVP, Jha B (2008) Isotherm modeling for biosorption of Cu(II) and Ni(II) from wastewater onto brown seaweed, Cystoseira indica. AIChE J 54:3291–3302. doi:10.1002/aic.11606
Basha S, Jaiswar S, Jha B (2010) On the biosorption, by brown seaweed, Lobophora variegata, of Ni(II) from aqueous solutions: equilibrium and thermodynamic studies. Biodegradation 21:661–680. doi:10.1007/s10532-010-9333-4
Bhatnagar A, Vilar VJP, Ferreira C, Botelho CMS, Boaventura RAR (2012) Optimization of nickel biosorption by chemically modified brown macroalgae (Pelvetia canaliculata). Chem Eng J 193–194:256–266. doi:10.1016/j.cej.2012.04.037
Bilal M, Shah JA, Ashfaq T, Gardazi SMH, Tahir AA, Pervez A et al (2013) Waste biomass adsorbents for copper removal from industrial wastewater—a review. J Hazard Mater 263:322–333. doi:10.1016/j.jhazmat.2013.07.071
Boyd GE, Soldano BA (1953) Self-diffusion of cations in and through sulfonated polystyrene cation-exchange polymers. J Am Chem Soc 75:6091–6099. doi:10.1021/ja01120a001
Boyd GE, Adamson AW, Myers LS (1947) The Exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics1. J Am Chem Soc 69:2836–2848. doi:10.1021/ja01203a066
Carpenter KE, Niem VH (1998) FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 1. Seaweeds, corals, bivalves and gastropods. FAO, Rome
Chan LS, Cheung WH, Allen SJ, McKay G (2012) Error analysis of adsorption isotherm models for acid dyes onto bamboo derived activated carbon. Chin J Chem Eng 20:535–542. doi:10.1016/S1004-9541(11)60216-4
Chien SH, Clayton WR (1980) Application of elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci Soc Am J 44:265–268. doi:10.2136/sssaj1980.03615995004400020013x
Chowdhury P, Elkamel A, Ray AK (2015) Photocatalytic processes for the removal of toxic metal ions. In: Sharma SK (ed) Heavy metals in water: presence, removal and safety. The Royal Society of Chemistry, UK, pp 25–43
de Souza FB, de Souza SMAG, de Souza AAU, Costa CE, Botelho CS, Vilar VP, Boaventura RR (2013) Modeling of trivalent chromium speciation in binding sites of marine macroalgae Sargassum cymosum. Clean Technol Environ Policy 15:987–997. doi:10.1007/s10098-012-0573-3
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–241. doi:10.1021/cr60204a006
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of the activated charcoal. Chem Zentralbl 1:875–890
Foo KY, Hameed BH (2013) Utilization of oil palm biodiesel solid residue as renewable sources for preparation of granular activated carbon by microwave induced KOH activation. Bioresour Technol 130:696–702. doi:10.1016/j.biortech.2012.11.146
Freile-Pelegrín Y, Robledo D, Azamar JA (2006) Carrageenan of Eucheuma isiforme (Solieriaceae, Rhodophyta) from Yucatán, Mexico. I. Effect of extraction conditions. Bot Mar 49:65–71. doi:10.1515/BOT.2006.008
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem Leipz 57:385–470
Gautam RK, Sharma SK, Mahiya S, Chattopadhyaya MC (2015) Contamination of heavy metals in aquatic media: transport, toxicity and technologies for remediation. In: Sharma SK (ed) Heavy metals in water: presence, removal and safety. The Royal Society of Chemistry, UK, pp 1–24
Gibbs JW (1873) A method of geometrical representation of the thermodynamic properties of substances by means of surfaces. Trans Conn Acad Arts Sci 2:382–404
Grassi M, Kaykioglu G, Belgiorno V, Lofrano G (2012) Removal of emerging contaminants from water and wastewater by adsorption process. In: Lofrano G (ed) Emerging compounds removal from wastewater. Springer, Netherlands, pp 15–37
Gupta SS, Bhattacharyya KG (2011) Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Colloid Interface Sci 162:39–58. doi:10.1016/j.cis.2010.12.004
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78. doi:10.1016/j.biortech.2014.01.068
He J, Hong S, Zhang L, Gan F, Ho YS (2010) Equilibrium and thermodynamic parameters of adsorption of methylene blue onto rectorite. Fresenius Environ Bull 19:2651–2656
Ho YS (1995) Adsorption of heavy metals from waste streams by peat. Dissertation, University of Birmingham
Ho Y-S (2004) Selection of optimum sorption isotherm. Carbon 42:2115–2116. doi:10.1016/j.carbon.2004.03.019
Ho YS, Ofomaja AE (2006) Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J Hazard Mater 129:137–142. doi:10.1016/j.jhazmat.2005.08.020
Hobson JP (1969) Physical adsorption isotherms extending from ultrahigh vacuum to vapor pressure. J Phys Chem 73:2720–2727. doi:10.1021/j100842a045
Ibrahim WM (2011) Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazard Mater 192:1827–1835. doi:10.1016/j.jhazmat.2011.07.019
Karim AH, Jalil AA, Triwahyono S, Kamarudin NHN, Salleh NFM, Aziz MAA (2012) Adsorption of methylene blue from aqueous solution by mesostructured silica nanoparticles. In: Proceedings of the 5th international conference on postgraduate education (ICPE-5 2012), 18–19 December 2012, Universiti Technologi Malaysia, Malaysia
Kumar PS, Saravanan A, Rajan PS, Yashwanthraj M (2016) Nanoscale zero-valent iron-impregnated agricultural waste as an effective biosorbent for the removal of heavy metal ions from wastewater. Text Cloth Sustai 2:3. doi:10.1186/s40689-016-0014-5
Lagergren SY (1898) Zur Theorie der sogenannten Adsorption gelöster Stoffe. Kongl Svenska Vetensk Akad Handl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. doi:10.1021/ja02242a004
Lasheen MR, Ammar NS, Ibrahim HS (2012) Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: equilibrium and kinetic studies. Solid State Sci 14:202–210. doi:10.1016/j.solidstatesciences.2011.11.029
Leo WR (1994) Techniques for nuclear and particle physics experiments: a how-to approach, 2nd edn. Springer, Berlin, pp 81–113
Lezcano JM, González F, Ballester A, Blázquez ML, Muñoz JA, García-Balboa C (2010) Biosorption of Cd(II), Cu(II), Ni(II), Pb(II) and Zn(II) using different residual biomass. Chem Ecol 26:1–17. doi:10.1080/02757540903468102
Macek T, Mackova M (2011) Potential of biosorption technology. In: Kotrba P, Mackova M, Macek T (eds) Microbial biosorption of metals. Springer, Netherlands, pp 7–17
Maksin DD, Kljajević SO, Đolić MB, Marković JP, Ekmeščić BM, Onjia AE, Nastasović AB (2012) Kinetic modeling of heavy metal sorption by vinyl pyridine based copolymer. Hem Ind 66:795–804. doi:10.2298/HEMIND121002112M
Massocatto CL, Paschoal EC, Buzinaro N, Oliveria TF, Tarley CRT, Caetano J et al (2013) Preparation and evaluation of kinetics and thermodynamics studies of lead adsorption onto chemically modified banana peels. Desalin Water Treat 51:5682–5691. doi:10.1080/19443994.2013.770614
Meitei MD, Prasad MNV (2014) Adsorption of Cu (II), Mn(II) and Zn (II) by Spirodela polyrhiza (L.) Schleiden: equilibrium, kinetic and thermodynamic studies. Ecol Eng 71:308–317. doi:10.1016/j.ecoleng.2014.07.036
Ncibi MC, Altenor S, Seffen M, Brouers F, Gaspard S (2008) Modelling single compound adsorption onto porous and non-porous sorbents using a deformed Weibull exponential isotherm. Chem Eng J 145:196–202. doi:10.1016/j.cej.2008.04.001
Nessim RB, Bassiouny AR, Zaki HR, Moawad MN, Kandeel KM (2011) Biosorption of lead and cadmium using marine algae. Chem Ecol 27:579–594. doi:10.1080/02757540.2011.607439
Park D, Yun YS, Park J (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15:86–102. doi:10.1007/s12257-009-0199-4
Pauling L (1960) The nature of the chemical bond: an introduction to modern structural chemistry, 3rd edn. Cornell University Press, Ithaca
Pennesi C, Totti C, Romagnoli T, Bianco B, De Michelis I, Beolchini F (2012) Marine macrophytes as effective lead biosorbents. Water Environ Res 84:9–16. doi:10.2175/106143011X12989211841296
Perales YJ, Leysa M (2012) Eucheuma denticulatom as potential biosorbent for lead nitrate, cadmium sulfide and zinc sulfate contaminated waters. Int Proc Chem Biol Environ Eng 45:117–120. doi:10.7763/IPCBEE.2012.V45.24
Plaza Cazón J, Viera M, Donati E, Guibal E (2013) Zinc and cadmium removal by biosorption on Undaria pinnatifida in batch and continuous processes. J Environ Manag 129:423–434. doi:10.1016/j.jenvman.2013.07.011
Plazinski W, Plazinska A (2012) Equilibrium and kinetic modeling of adsorption at solid/solution interface. In: Bhatnagar A (ed) Application of adsorbents for water pollution control. Bentham Science Publishers, The Netherlands, pp 32–80
Rathod M, Mody K, Basha S (2014) Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alvarezii. J Clean Prod 84:484–493. doi:10.1016/j.jclepro.2014.03.064
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75:589–597. doi:10.1021/ja01099a022
Renaud S, Luong-Van J (2006) Seasonal variation in the chemical composition of tropical Australian marine macroalgae. J Appl Phycol 18:381–387. doi:10.1007/s10811-006-9034-x
Roginsky SZ, Zeldovich YB (1934) Die katalische Oxidation von Kohlenmonoxyd auf Mangandioxyd. Acta Physiochim USSR 1:554–594
Sarkar A, Bhagat J, Sarker S (2014) Evaluation of impairment of DNA in marine gastropod, Morula granulata as a biomarker of marine pollution. Ecotox Environ Safe 106:253–261. doi:10.1016/j.ecoenv.2014.04.023
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141. doi:10.1016/j.jcis.2004.01.036
Sherwood AR, Kurihara A, Conklin KY, Sauvage T, Presting GG (2010) The Hawaiian Rhodophyta Biodiversity Survey (2006-2010): a summary of principal findings. BMC Plant Biol 10:258. doi:10.1186/1471-2229-10-258
Sreńscek-Nazzal J, Narkiewicz U, Morawski AW, Wróbel RJ, Michalkiewicz B (2015) Comparison of optimized isotherm models and error functions for carbon dioxide adsorption on activated carbon. Pol J Chem Eng Data 60:3148–3158. doi:10.1021/acs.jced.5b00294
Su Y, Zhao B, **ao W, Han R (2013) Adsorption behavior of light green anionic dye using cationic surfactant-modified wheat straw in batch and column mode. Environ Sci Pollut Res 20:5558–5568. doi:10.1007/s11356-013-1571-7
Subramanyam B, Das A (2014) Linearised and non-linearised isotherm models optimization analysis by error functions and statistical means. J Environ Health Sci Eng 12:92. doi:10.1186/2052-336X-12-92
Temkin MJ, Pyzhev V (1940) Recent modifications to Langmuir isotherms. Acta Physiochim USSR 12:217–222
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133:304–308. doi:10.1016/j.jhazmat.2005.10.016
Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C (2010) Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Bioresour Technol 101:5808–5814. doi:10.1016/j.biortech.2010.02.099
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238. doi:10.1002/aic.690200204
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Environ Eng ASCE 89:31–60
Yalçın S (2014) The mechanism of heavy metal biosorption on green marine macroalga Enteromorpha linza. CLEAN 42:251–259. doi:10.1002/clen.201200500
Yalçın S, Sezer S, Apak R (2012) Characterization and lead(II), cadmium(II), nickel(II) biosorption of dried marine brown macro algae Cystoseira barbata. Environ Sci Pollut Res 19:3118–3125. doi:10.1007/s11356-012-0807-2
Acknowledgments
The authors greatly acknowledge the financial support of the AIMST University (Grant Number: AURGC/1/FAS/2013) for the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.S., Sathasivam, K.V. Heavy metal biosorption potential of a Malaysian Rhodophyte (Eucheuma denticulatum) from aqueous solutions. Int. J. Environ. Sci. Technol. 13, 1973–1988 (2016). https://doi.org/10.1007/s13762-016-1022-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1022-3