Abstract
The characterization of swelling clays is important for diverse fields, including the field of conservation of built cultural heritage. Villarlod molasse, a building stone utilized frequently across Switzerland, is known to be damaged by swelling clays embedded in its matrix. In this study, the mechanism of how the clays lead to swelling in the stone itself is examined, and similar to previous studies, crystalline swelling is noted as the most likely source. A scaling factor linking X-ray diffraction (XRD) and dilatometric swelling experiments is calculated, and evidence for the existence of an initial monolayer of moisture in the embedded clays at ambient relative humidities is presented. A qualitative micromechanical model describing how the nonswelling stone matrix exerts a pressure on the clay layers, affecting their swelling behavior, is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swelling clay minerals are of interest for diverse areas of geotechnical engineering, in particular stability of slopes, foundations, tunnels, and boreholes (O’Neill and Poormoayed 1980; Anagnostou et al. 2010; Anderson et al. 2010; Khan et al. 2017). They are of particular interest as well in the field of conservation of built cultural heritage, as they often appear in the matrix of sandstones and other stones used in historic buildings and monuments, where wetting and drying cycles can lead to damage (Rodriguez-Navarro et al. 1997; Sebastián et al. 2008; Ruedrich et al. 2011; Wedekind et al. 2013; Berthonneau et al. 2016; Pötzl et al. 2018; Elert and Rodriguez-Navarro 2022; Siegesmund et al. 2023). This damage was proposed to come from differential expansion and contraction of the whole stone leading to strain gradients, with damage from wetting cycles leading to buckling failure (also called spalling, contour scaling), and damage from drying cycles leading to mud cracking failure (Gonzalez and Scherer 2004; Jiménez-González et al. 2008). Buckling failure, a type of surface delamination more often seen in the field than mud cracking, was shown to require a flaw propagation mechanism to reach a critical failure point (Wangler et al. 2011). Recent studies have shown the importance of anisotropy with respect to observed failure mechanisms (Tiennot et al. 2017), and, more critically, the microstructural role that the clays play in the actual fracture process (Tiennot et al. 2019). Even more recently, the synergy between salt damage and clay swelling damage has been studied (Taye et al. 2022). Finally, evidence that microcrack formation and propagation simply from the swelling clays themselves can lead to permanent deformation was demonstrated in an extensive study by Elert et al. in tuffs from Copan (2021) as well as by Wangler and Sanchez in Villarlod molasse (2020). For a recent review on the general topic of swelling clays and damage in heritage sandstones, the reader is referred to Elert and Rodriguez-Navarro (2022).
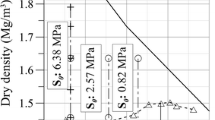
The mechanism of swelling in the swelling clay minerals, or smectites, has its origin in the structure of the smectites. Two tetrahedral silica sheets sandwich an octahedral sheet in a 2:1 configuration, and random substitutions of atoms of lower valence, primarily in the octahedral sheet, lead to a net negative charge in the 2:1 structure (Olphen 1977). This net negative charge is balanced by counterbalancing cations that exist between each of these sheets, and subsequent hydration and dehydration of these ions and the negatively charged silica sheets leads to an overall expansion and contraction of the structure. This occurs over two distinct ranges as measured by X-ray diffraction (XRD): (1) crystalline swelling, where layers of water hydrate the cations leading to discrete increases in the d-spacing, and (2) osmotic swelling, a more continuous expansion where the driving force for swelling is a concentration gradient between the clay sheets and the bulk pore solution (Norrish 1954; Olphen 1977; Madsen and Müller-Vonmoos 1989). It is worth noting that osmotic swelling pressures develop via DLVO interactions, and can thus occur also between nonswelling clays, such as illites. In the case of heritage stones, other authors have suggested the possibility of disjoining pressures also leading to swelling (Ruedrich et al. 2011) in certain stones which may not contain any clays. Previous results suggest that even upon exposure to ambient relative humidities, sandstones with clays are swelling primarily through crystalline swelling (Wangler and Scherer 2008; Colas et al. 2011; Wangler 2016). More specifically, in the first of these studies it was demonstrated that in three American sandstones, including Portland brownstone, the swelling strain could be directly related to the d-spacing as measured by XRD, as depicted in Fig. 1.
Relationship between swelling strain and clay moisture content in Portland Brownstone and Pennsylvania Bluestone, based on Wangler and Scherer (Wangler and Scherer 2008)
Of particular note is the role of the microstructure in swelling of stones. Clays that exist between grains are of far more consequence than clays found within pockets, and this has large consequences for the mechanical behavior of the stones when either wet or dry (Gonzalez and Scherer 2004; Ruedrich et al. 2011; Tiennot et al. 2019).
Villarlod molasse is a stone of high importance to the cultural heritage of Switzerland, and variants of the molasses in the Swiss plateau are widespread throughout Switzerland and southwest Germany. The molasse has been noted for poor weathering performance, mostly attributed to the generally high swelling clay content (Félix 1994; Gonzalez and Scherer 2004). Recent studies on molasse have focused on the details of its fracture mechanism (Tiennot and Bourgès 2016) and on-site performance (Demoulin et al. 2016; Praticò et al. 2020). The swelling clays have also been pointed out as problematic in application of consolidation strategies for this stone, rendering treatments ineffective after just one or two wetting cycles, a problem noted in other stones as well (Elert et al. 2021). Thus understanding the swelling behavior and how it leads to damage is of prime importance for the built cultural heritage of Switzerland. In this study, the focus is primarily on the details of the swelling mechanism, and a similar approach is taken as the study of Wangler and Scherer (2008) to uncover the relationship between d-spacing and swelling strain, as seen in Fig. 1, for Villarlod molasse.
Materials and methods
Materials
Stone
The stone used in this study was Villarlod molasse, a clay-rich subarkose sandstone quarried from Carrière de Molasse de Villarlod, Farvagny, Switzerland. Stone is cut from the quarry in large blocks, then samples are cut for swelling experiments using diamond saws down to prisms 5 cm in height and 5 × 5 mm in cross section. The swelling strain in water of the Villarlod molasse used in this study was 1.8 mm/m. The anisotropy of the stone was taken into consideration, and for all samples the bedding planes were oriented perpendicular to the swelling direction.
Cation pretreatments
For cation pretreatments, solutions of 2.0 M were prepared of various chloride salts (Na+, K+, Mg2+, and Ca2+). The stone samples were pretreated by immersion of the dry samples in the solutions for 12 h, then transferred to ca. 1 L stirred deionized water in beakers for 18–24 h. Considering sample sizes and porosity, a dilution factor of ca. 4000 can be estimated at equilibrium. It was assumed that equilibrium was reached, as the concentrated salt solution had more than adequate diffusion time out of the samples, with a diffusion length of only 2.5 mm.
Clay fraction
The clay fraction from the Villarlod molasse was separated according to USGS OFR 01–041 (Poppe et al. 2000). The procedure includes first a manual grinding, followed by an acetic acid digestion of the carbonate phases. The remaining solid is then put through a series of centrifugation steps until only the clay sized fraction (< 2 mm) remains suspended. This suspension is then flocculated with a 1.0 M CaCl2 solution and centrifuged, with the solid fraction dried in an oven.
Experimental methods
X-ray diffraction (XRD)
XRD experiments were all performed on a Bruker AXS D8 ADVANCE diffractometer (Bruker Corporation, Karlsruhe, Germany) with CoKα (λ = 1.7902 Å) radiation. Two types of experiments were performed: quantitative Rietveld analysis of the whole stone, and d-spacing measurements on the oriented clay fraction under various conditions.
Rietveld analysis, reported elsewhere (Wangler 2016), was performed on a randomly oriented sample of stone powder, ground and sieved to < 20 µm and placed in a front-loading sample holder, using a razor blade to reduce preferred orientation. Scanning was performed between 2° and 80° 2θ at step sizes of 0.02° and 4 s per step. Qualitative analysis to identify peaks was performed using DIFFRACPlus EVA software by matching peak locations and intensities to the database of the International Centre for Diffraction Data (ICDD). AutoQuan software (GE Inspection Technologies) was performed for quantitative mineralogical phase analysis, using Rietveld refinement to adapt phase contents and phase-specific parameters to minimize the difference between a calculated and the measured diffractogram.
Oriented clay fraction XRD measurements were performed by first applying a thin slurry of the clay-sized fraction on a glass slide and then allowing it to dry. Glass slides were then treated using various solvents: water and methanol, applied by an eyedropper directly before scanning, and ethylene glycol and 1,3 propanediol, applied by placing the slides in a desiccator with each of these solvents, overnight, in a 60 ℃ oven. Diffractograms were performed from 1° to 30° 2θ at 0.02° steps, with 1 s dwell time. The scan time of about 25 min was too long to allow measurement of the methanol spacing due to its volatility, and thus this is excluded from the results.
Exchangeable cations
These experiments were carried out using a procedure adapted from Meier (1999). A copper pentaethylenehexamine (Cu pent(en)) solution of 0.01 M was prepared from copper sulfate and pentaethylenehexamine. The solution was then contacted with a measured amount of the stone that had been crushed by a mortar and pestle and allowed to equilibrate in an agitated suspension for 1 h. The suspension was then centrifuged and decanted. The supernatant was diluted 1:100 with a 2wt% HNO3 solution before analysis by inductively coupled plasma optical emission spectroscopy (ICP-OES) for the following ions, expected to be the dominant exchangeable species: K+, Na+, Ca2+, and Mg2+.
Swelling experiments
Swelling experiments were carried out with a Linear Variable Differential Transducer (LVDT) obtained from TESAGroup (France) with a resolution of ± 0.2 μm. The LVDT tip was contacted to the surface of the sample in a small stainless steel cup, which was then filled with water or an organic solvent. The swelling strain was measured by dividing the measured linear deformation by the length of the sample after approximately 60–90 min, which is more than enough time for samples of this dimension to fully saturate. In one experiment, the water or solution surrounding the sample was suctioned out and either replaced with another solution, or the sample was dried with a compressed air gun.
Results
The Rietveld analysis of Villarlod molasse is seen in Table 1. The swelling clay fraction is on the order of 5–6%, and also noteworthy is the carbonate fraction of the rock, with calcite at 17% and dolomite at 3%.
The exchangeable cations as measured by the release of Ca2+, Mg2+, K+, and Na+ are seen in Table 2. The dominant exchangeable cations of these four are Ca2+ and K+.
Table 3 shows the results of the swelling experiments with cation pretreatments. Noteworthy results from this include the relatively lower amounts of swelling observed with K+ and Mg2+. A higher swelling amount is observed with Na+, while Ca2+ swelling strain is more in line with that observed for the untreated stone.
Figure 2 shows the swelling strains for various organic solvents, while Fig. 3 shows the corresponding XRD results. The drying rate of methanol was too high to allow for the accurate measurement of the d-spacing of the clay fraction with methanol, therefore a reference value of 14.1. Å taken from German and Harding (1971) is taken. One can see that ethylene glycol’s layer spacing is 16.5 Å, a bit lower than the usual basal spacings observed in swelling clays (16.9–17.1 Å), but lower ethylene glycol basal spacings have been noted in higher layer charge swelling clay minerals (Brindley 1966). Difficulties in timing the drying of the water saturated clay fraction made a good scan difficult to procure, but a diffuse peak towards 19 Å (indicative of the upper limit of Ca-montmorillonite swelling) is observed.
Discussion
In the earlier study of Wangler and Scherer (2008), it was shown that in three separate sandstones, swelling can be attributed almost exclusively to crystalline swelling of the clay minerals, and a scaling factor (here named as SF) was developed relating swelling strain measured by dilatometry, and d-spacing obtained from XRD:
where εs and d001 are the measured swelling strains and d-spacings at different conditions. With this relationship, one could easily determine the d-spacing of the clays confined within the stone with a simple dilatometric experiment. This relationship can be modified to
where d001,initial corresponds to the initial d-spacing before a swelling experiment, and εs and d001,final correspond to the measured swelling strain and final d-spacing, respectively, for a given swelling experiment.
This relationship arose due to the direct comparison of XRD and dilatometric experiments, which showed an approximate doubling of the swelling strain when comparing methanol to ethylene glycol swelling. This corresponded to an approximate doubling of the interlayer space from an increase of 4.1 Å (methanol) to 7.1 Å (ethylene glycol). A key conclusion from this study was that in addition to the swelling range apparently being entirely crystalline, the initial d-spacing before any kind of wetting in the stones of the study (εs,1 = 0) was 10 Å, which corresponds to a completely dehydrated interlayer. This result was unexpected, as usually, there are one or more layers of water in a clay interlayer at ambient laboratory relative humidities (40–60%); for example, most studies show a bilayer at ambient relative humidities in Ca-montmorillonite (Méring 1949; Tamura et al. 2000). The dehydrated interlayer was hypothesized to be due to the fact that the rock matrix exerts a pressure on the clay interlayers at grain interstices, forcing the water out.
The swelling behavior described in the previous paragraph is not observed in this study with Villarlod molasse, however. The ethylene glycol swelling strain is almost triple that of the methanol swelling strain, which does not correspond proportionally to an increase in interlayer spacing from an initially dry clay interlayer. However, when one assumes that there is a monolayer of water in the clay interlayer, then the proportional increases that are subsequently calculated make sense. From Figs. 2 and 3, if one assumes an initial layer spacing of approximately 12.5 Å, then one can see that the increase to 14.1 Å for methanol, and 16.5 Å for ethylene glycol (as well as the spacing of 14.8 Å for 1,3 propanediol) can then be related well to their respective swelling strains through (2). For example, the relative increase for methanol would be 1.6 Å, corresponding to 0.42 mm/m in swelling, giving approximately 0.26 mm/m-Å as a scaling factor relating the two. A similar analysis for ethylene glycol gives 0.27 mm/m-Å. This is summed up in Table 4, and leads to a similar conclusion to the study of Wangler & Scherer (2008), that swelling in this particular stone is predominantly crystalline in nature. It is clear to see in Table 4 that assuming a completely dry interlayer leads to a systematic error, which is immediately corrected when one assumes that there is a single monolayer of water in the interlayer. The agreement among the four solvents that this correction makes gives very compelling evidence for the accuracy of the scaling factor, as well as the accuracy of the assumption of the initial d-spacing. Considering the difficulty in measuring the actual initial d-spacing within the stone due to the relatively low clay contents, this method could be quite useful. However, this rests on the assumption that the swelling behavior of the clay within the stone while saturated with each solvent is the same as the saturated swelling behavior outside of the stone during an XRD experiment.
Irrespective of the saturated swelling behavior, it is clear, however, that the d-spacing of the clay interlayers at ambient RH when measured outside of the stone mass (14.8 Å, Fig. 3) and the calculated d-spacing at ambient RH when within the stone mass (~ 12.5 Å) do not match. This supports the earlier evidence from Wangler & Scherer (2008) that there is an external pressure applied from the rock mass on the clay interlayers, acting to keep water out at ambient RH. Evidently, in the case of Villarlod molasse, this external pressure is just high enough to keep out a bilayer of water, but a monolayer is possible. Ambient RH is generally in the range of 40–60%, and experimental results show that Ca-montmorillonite converts from a monolayer to a bilayer at RH no higher than 10% (Tamura et al. 2000), so the external pressure is apparently lowering the effective interlayer RH to < 10%.
Building upon the idea that the stone mass creates an external pressure on the clays, a microstructural model of the stone can be imagined in which the sandstone consists of grains where some have swelling clays, and some do not, and as described earlier, the nonswelling grain junctions exert a stress on the grain junctions that swell, as seen in Fig. 4a. The nonswelling junctions can be imagined as a spring, as depicted in Fig. 4b for the case of Villarlod molasse, and this spring exerts the opposing stress for the entry of water into the clay interlayer. This stress is apparently not enough to remove the final interlayer of water in Villarlod molasse at ambient relative humidities. Additionally, this stress also does not seem to play a large role in the saturated state using various solvents, as described earlier, although further work will need to confirm this.
The results of the cation pretreatment experiments support the implication of the previous analysis, in that swelling of Villarlod molasse with K+, which forms an “inner sphere” hydrate, has a significantly reduced swelling strain. This corresponds to earlier observations of lower d-spacings in K-saturated clays in the wet state (MacEwan and Wilson 1980). The behavior of the untreated stone compared to stone pretreated with Ca2+ leads one to believe that Ca2+ is most likely the predominant cation, which is reasonable to expect considering the calcareous matrix. The results of the exchangeable cation analysis (Table 2), however, show a significant proportion of potassium as the exchangeable cation. This likely means that calcium and potassium are intermixed in the interlayers, with calcium dominating the swelling behavior. The potassium interlayer cation source is possibly altered microcline (Saigal et al. 1988), as both XRD and SEM–EDX show significant amounts of microcline and plagioclase albite (Wangler 2016; Wangler and Sanchez 2020). In any case, this underscores the importance of the cation species in understanding the behavior of swelling clay minerals in sandstones.
With the scaling factor of 0.27 mm/m-Å, one can then easily calculate relative layer spacing increases for various cations based on their swelling strains in water, summarized in Table 5.
One can see that some layer spacing changes are very large, such as sodium’s, and some are very small, such as potassium’s. Crystalline swelling does not really proceed above a 10 Å layer spacing increase (Madsen and Müller-Vonmoos 1989), and therefore some of these calculated layer spacing increases result from either a lower initial layer spacing (indicating some water has been forced out) or a certain degree of osmotic swelling, or both. Looking at the case of potassium, the layer spacing increase of about 2.4 Å indicates a monolayer of water is entering the interlayer. However, whether this is happening from an initially dry state (and thus the layer is the first monolayer) or from the monolayer to the bilayer state, cannot be determined from these data. Concerning the higher increase in the sodium treated stone, it is possible that this range corresponds to the full range allowable by a dehydrated interlayer, because a monolayer spacing of water added to the calculated interlayer spacing change is 10.5 Å, which is a bit above the upper limit for crystalline swelling. This would mean that similar to the sandstones studied by Wangler and Scherer (2008), the rock matrix is able to successfully push out the monolayer of water at ambient relative humidities. Considering that the hydration energy of Na+ is weaker than Ca2+, this is a plausible explanation, but it does not rule out the possibility that there is already a monolayer of water and there is a certain amount of osmotic swelling occurring. It is also interesting to note that apparently Mg2+ has initially a bilayer of water in the interlayer at ambient conditions. This contrasts with the behavior of Ca2+, also divalent. This could be because of the stronger hydration energy of Mg2+ relative to Ca2+, which would attract a bilayer of moisture at ambient relative humidities (Salles et al. 2013). In any case, further studies with solvents and cation pretreatments should lead to a better understanding, in particular on the initial d-spacing.
It bears noting that the degree of exchange in the clay of the various cation pretreatments is unknown. It is therefore quite possible that in any of the experiments, there is an incomplete degree of exchange, and thus the swelling behavior is reflective of mixed interlayers. However, one can state that the ion pretreatments have a large effect on the swelling behavior, which again points to crystalline swelling being the primary swelling mode. Additionally, general trends such as those noted above can be determined as in agreement with what is known on cation effect on crystalline swelling in smectites.
An interesting experiment to confirm the existence of interlayer water at ambient conditions was carried out, in which a dilatometric experiment was carried out by swelling initially with water, then replacing the surrounding fluid with a 1.0 M KCl solution, followed by drying and then rewetting. This experiment is seen in Fig. 5, in which a Villarlod molasse stone with an Na+ pretreatment is subjected to this procedure.
Experiment in which a Na+ treated sample of Villarlod molasse is first swelled with DI water (1), then the water is suctioned out and replaced by a 1.0 M KCl solution (2) which diffuses into the sample and causes a contraction. Subsequent drying (3) then causes the sample to contract to a length shorter than its initial length, indicating a change in initial interlayer spacing in the clay. Upon subsequent rewetting (4) the sample swells to approximately the swelling strain of a K+ treated sample
In this experiment, one can observe the initial swelling with water followed by a contraction as the potassium diffuses into the sample and into the clay interlayers. Upon drying, the sample then contracts, as expected, but it contracts to a dimension less than its initial dimension, indicating a contraction of the clay interlayers relative to the initial d-spacing. This experiment provides compelling evidence that there is some moisture in the interlayer initially, and that the (full or partial) exchange of a weaker hydration energy cation (K+) allows the matrix of the rock to remove this interlayer water when drying occurs. This is noteworthy, as earlier it was suggested that sodium might allow the moisture in the interlayer to dehydrate fully at ambient RH, but this is apparently not the case. Finally, subsequent wetting then matches the measured swelling strain of the potassium treated stone in Table 3 of approximately 0.65 mm/m.
There are some implications of this work for the conservation of built cultural heritage constructed with Villarlod molasse, primarily when it comes to understanding and mitigating the swelling behavior. The fact that the swelling is predominantly crystalline points again to treatment strategies that target this, such as ion exchange with species that induce lower swelling. The most effective anti-swelling agents to date have been the α, ω alkyldiamines, first tested by Snethlage and Wendler (1990) and the mechanism elucidated by Wangler and Scherer (2009). The mechanism of swelling reduction in Villarlod molasse, performed similarly to the previously cited study of Wangler and Scherer, will be the subject of another study. However, the possibilities of a species such as potassium, which is much cheaper and appears to be able to reduce swelling significantly, have not yet been explored in the context of cultural heritage. Besides understanding its efficacy, however, the use of potassium must also be examined in terms of the amount required and the durability of the treatment. A treatment such as this, however, or a proven one such as the alkyldiamine treatment, could prove useful if done as a pretreatment before a restoration.
Conclusion
The results of this study demonstrate that for Villarlod molasse:
-
The clays responsible for moisture expansion are swelling mostly by crystalline swelling, although other swelling modes could contribute as well, particularly osmotic swelling in certain circumstances. This corresponds closely with earlier results suggesting that in many sandstones with swelling clays, crystalline swelling is the dominant mode.
-
The stone swelling strain can be linked to the clay swelling behavior on the Å scale, with a scaling factor calculated at 0.27 mm/m-Å. This can be utilized to calculate changes in the clay interlayer spacing via simple swelling experiments. It should be cautioned that clay content may vary within stone taken from the same formation, so the scaling factor calculated here is not necessarily fixed, and experiments performed should serve as a framework for characterizing swelling in Villarlod molasse and other stones.
-
At ambient relative humidities, evidence points to a monolayer of water existing in the clay interlayer within the stone matrix, while a bilayer exists in the separated clay fraction. A proposed hypothesis for this discrepancy is the stress imposed on the clay interlayer by the rock matrix, which forces out the water.
-
The interlayer cation makeup appears to be primarily Ca2+ and K+, with Ca2+ apparently dominating the swelling behavior. The interlayer cation has a very large effect on the swelling behavior, as one would expect with crystalline swelling being the dominant mode.
Further points of investigation then can be recommended:
-
The role of external pressure on a swelling clay interlayer should be further investigated, especially as it relates to variable moisture contents. XRD, for example using synchrotron radiation, of the clay at various moisture states while it is in the stone would provide direct experimental proof of a dry, mono-, or bi-layer of water. Additionally, the combination of moderate external pressures and lower relative humidities has not been studied a lot, and this unique environment exists in a stone matrix at relatively dry (ambient) conditions.
-
The micromechanical model of clays at interlayer junctions and their impact on global mechanical properties can be further investigated and developed, which will be the topic of further study.
-
The common swelling inhibitors used in cultural heritage conservation (and also used in swelling inhibition for drilling borehole stability) can be studied using the techniques and relationships developed in this study. This will be the topic of another study for the specific case of Villarlod molasse and alkyldiamines.
Data availability
The author will provide data for this study upon request.
References
Anagnostou G, Pimentel E, Serafeimidis K (2010) Swelling of sulphatic claystones—some fundamental questions and their practical relevance. Quellen von sulfatführenden Tonsteinen—Themen der Grundlagenforschung und ihre praktische Bedeutung. Geomech Tunn 3:567–572. https://doi.org/10.1002/geot.201000033
Anderson RL, Ratcliffe I, Greenwell HC et al (2010) Clay swelling—a challenge in the oilfield. Earth-Sci Rev 98:201–216. https://doi.org/10.1016/j.earscirev.2009.11.003
Berthonneau J, Bromblet P, Cherblanc F et al (2016) The spalling decay of building bioclastic limestones of Provence (South East of France): from clay minerals swelling to hydric dilation. J Cult Herit 17:53–60. https://doi.org/10.1016/j.culher.2015.05.004
Brindley GW (1966) Ethylene glycol and glycerol complexes of smectites and vermiculites. Clay Miner 6:237–259. https://doi.org/10.1180/claymin.1966.006.4.01
Colas E, Mertz JD, Thomachot-Schneider C et al (2011) Influence of the clay coating properties on the dilation behavior of sandstones. Appl Clay Sci 52:245–252. https://doi.org/10.1016/j.clay.2011.02.026
Demoulin T, Girardet F, Wangler TP et al (2016) On-site monitoring for better selection of stone repairs: a case study. Herit Sci 4:38. https://doi.org/10.1186/s40494-016-0108-z
Elert K, Rodriguez-Navarro C (2022) Degradation and conservation of clay-containing stone: a review. Constr Build Mater 330:127226. https://doi.org/10.1016/j.conbuildmat.2022.127226
Elert K, Ruiz-Agudo E, Jroundi F et al (2021) Degradation of ancient Maya carved tuff stone at Copan and its bacterial bioconservation. Npj Mater Degrad 5:1–12. https://doi.org/10.1038/s41529-021-00191-4
Félix C (1994) Déformation des grès consecutive à leur consolidation avec un silicate d’ethyle. Balkema, Lisbon, pp 3543–3550
German WL (1971) Primary aliphatic alcohol-homoionic montmorillonite interactions. Clay Miner 9:167–175. https://doi.org/10.1180/claymin.1971.009.2.02
Gonzalez IJ, Scherer GW (2004) Effect of swelling inhibitors on the swelling and stress relaxation of clay bearing stones. Env Geol 46:364–377. https://doi.org/10.1007/s00254-004-1038-8
Jiménez-González I, Rodríguez-Navarro C, Scherer GW (2008) Role of clay minerals in the physicomechanical deterioration of sandstone. J Geophys Res. https://doi.org/10.1029/2007JF000845
Khan MS, Hossain S, Ahmed A, Faysal M (2017) Investigation of a shallow slope failure on expansive clay in Texas. Eng Geol 219:118–129. https://doi.org/10.1016/j.enggeo.2016.10.004
MacEwan DMC, Wilson MJ (1980) Interlayer and intercalation complexes of clay minerals. In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-ray identification. Mineralogical Society of Great Britain and Ireland, London
Madsen FT, Müller-Vonmoos M (1989) The swelling behaviour of clays. Appl Clay Sci 4:143–156. https://doi.org/10.1016/0169-1317(89)90005-7
Meier LP (1999) Determination of the cation exchange capacity (CEC) of clay minerals using the complexes of copper(II) ion with triethylenetetramine and tetraethylenepentamine. Clays Clay Miner 47:386–388. https://doi.org/10.1346/CCMN.1999.0470315
Méring J (1949) L’interférence des rayons X dans les systèmes à stratification désordonée. Acta Cryst 2:371–377. https://doi.org/10.1107/S0365110X49000977
Norrish K (1954) The swelling of montmorillonite. Discuss Faraday Soc 18:120. https://doi.org/10.1039/df9541800120
O’Neill MW, Poormoayed M (1980) Methodology for foundations on expansive clays. J Geotech Geoenviron Eng 106(12):1345–1367
Olphen HV (1977) An introduction to clay colloid chemistry, for clay technologists, geologists, and soil scientists. An introduction to clay colloid chemistry, for clay technologists, geologists, and soil scientists.
Poppe LJ, Paskevich VF, Hathaway JC, Blackwood DS (2000) U.S. Geological Survey Open-File Report 01–041. https://pubs.usgs.gov/of/2001/of01-041/index.htm. Accessed 5 Jul 2020
Pötzl C, Dohrmann R, Siegesmund S (2018) Clay swelling mechanism in tuff stones: an example of the Hilbersdorf Tuff from Chemnitz Germany. Environ Earth Sci 77:188. https://doi.org/10.1007/s12665-018-7345-2
Praticò Y, Girardet F, Flatt RJ (2020) New Insights on the causes of contour scaling in Swiss sandstones. In: Siegesmund S, Middendorf B (eds) Monument Future: decay and conservation of stone. Mitteldeutscher Verlag, Halle, pp 907–912
Rodriguez-Navarro C, Hansen E, Sebastian E, Ginell WS (1997) The role of clays in the decay of ancient Egyptian limestone sculptures. J Am Inst Conserv 36:151–163. https://doi.org/10.1179/019713697806373172
Ruedrich J, Bartelsen T, Dohrmann R, Siegesmund S (2011) Moisture expansion as a deterioration factor for sandstone used in buildings. Environ Earth Sci 63:1545–1564. https://doi.org/10.1007/s12665-010-0767-0
Saigal GC, Morad S, Bjorlykke K et al (1988) Diagenetic albitization of detrital K-feldspar in Jurassic, Lower Cretaceous, and Tertiary clastic reservoir rocks from offshore Norway; I, textures and origin. J Sediment Res 58:1003–1013. https://doi.org/10.1306/212F8EE5-2B24-11D7-8648000102C1865D
Salles F, Douillard J-M, Bildstein O et al (2013) Driving force for the hydration of the swelling clays: case of montmorillonites saturated with alkaline-earth cations. J Colloid Interf Sci 395:269–276. https://doi.org/10.1016/j.jcis.2012.12.050
Sebastián E, Cultrone G, Benavente D et al (2008) Swelling damage in clay-rich sandstones used in the church of San Mateo in Tarifa (Spain). J Cult Herit 9:66–76. https://doi.org/10.1016/j.culher.2007.09.002
Siegesmund S, Gross CJ, Dohrmann R et al (2023) Moisture expansion of tuff stones and sandstones. Environ Earth Sci 82:146. https://doi.org/10.1007/s12665-023-10809-2
Snethlage R, Wendler E (1990) Surfactants and adherent silicon resins—new protective agents for natural stone. MRS Proc. https://doi.org/10.1557/PROC-185-193
Tamura K, Yamada H, Nakazawa H (2000) Stepwise hydration of high-quality synthetic smectite with various cations. Clays Clay Miner 48:400–404. https://doi.org/10.1346/CCMN.2000.0480311
Taye B, Viles H, Zhang H (2022) Influence of salt (NaCl) on hydric and hygric dilatation of clay-rich rocks. J Cult Herit 58:137–145. https://doi.org/10.1016/j.culher.2022.09.024
Tiennot M, Bourgès A (2016) Evaluation of small core-based specimens for characterization of stone deterioration. Int J Rock Mech Min Sci 84:69–73. https://doi.org/10.1016/j.ijrmms.2016.02.001
Tiennot M, Mertz J-D, Bourgès A (2017) Influence of anisotropic microcracking due to swelling on the fracture toughness of a clay-bearing sandstone. Rock Mech Rock Eng 50:2861–2870. https://doi.org/10.1007/s00603-017-1273-4
Tiennot M, Mertz J-D, Bourgès A (2019) Influence of clay minerals nature on the hydromechanical and fracture behaviour of stones. Rock Mech Rock Eng 52:1599–1611. https://doi.org/10.1007/s00603-018-1672-1
Wangler T (2016) Swelling Clay and Swelling Clay Inhibition in Villarlod Molasse. Science and art: a future for stone. University of the West of Scotland, Paisley
Wangler TP, Sanchez AMA (2020) Potential damage mechanism in Swiss molasses. Monument future: decay and conservation of stone. Proceedings of the 14th International Congress on the Deterioration and conservation of stone. Mitteldeutscher Verlag, Halle
Wangler T, Scherer GW (2008) Clay swelling mechanism in clay-bearing sandstones. Environ Geol 56:529–534. https://doi.org/10.1007/s00254-008-1380-3
Wangler T, Scherer GW (2009) Clay swelling inhibition mechanism of α, ω-diaminoalkanes in Portland Brownstone. J Mater Res 24:1646–1652. https://doi.org/10.1557/jmr.2009.0190
Wangler TP, Stratulat A, Duffus P et al (2011) Flaw propagation and buckling in clay-bearing sandstones. Environ Earth Sci 63:1565–1572. https://doi.org/10.1007/s12665-010-0732-y
Wedekind W, López-Doncel R, Dohrmann R et al (2013) Weathering of volcanic tuff rocks caused by moisture expansion. Environ Earth Sci 69:1203–1224. https://doi.org/10.1007/s12665-012-2158-1
Acknowledgements
The author would like to thank Dr. Michael Plötze (ClayLab, Institute for Geotechnical Engineering, ETH Zürich) for his valuable insights and support in the use of the XRD equipment. The author would also like to thank Dr. Francesco Caruso and Dr. Sara Mantellato (Physical Chemistry of Building Materials, Institute for Building Materials, ETH Zürich) for carrying out the ICP-OES measurements.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich. Some of the work performed in this project was funded by Swiss Federal Roads Office (ASTRA) Project FG2012/001.
Author information
Authors and Affiliations
Contributions
All work performed by the sole author.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wangler, T. Mechanism of clay swelling in Villarlod molasse: a Swiss sandstone. Environ Earth Sci 82, 259 (2023). https://doi.org/10.1007/s12665-023-10954-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-10954-8