Abstract
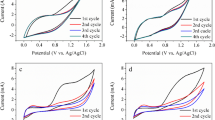
The emergence of antibiotics in aquatic environment has led to an increasing concern about the potential environmental risks and the spread of antibacterial resistance among microorganisms. Electrochemical oxidation processes are promising technologies to treat low contents of toxic and bio-refractory pollutants in water. Anodic oxidation of berberine, the frequently detected antibiotic in surface waters, was carried out by using RuO2/Ti, IrO2/Ti, RuIrO2/Ti, and Pt/Ti electrodes. It showed that with Pt/Ti anode, the removal efficiency of berberine was significantly higher than that with the other electrodes. Moreover, all the four electrodes showed 3–5 times higher reaction rate constant in NaCl solution than in Na2SO4 solution. The addition of chloride salts increased the oxidation rate of berberines due to the formation of active chlorines. In the case of Pt/Ti anode and simulated berberine wastewater, the effects of applied anodic bias, initial pH, Cl− and berberine concentrations, and reaction time on the removal efficiency of berberines were investigated. Under the optimal conditions of applied bias of 2.0 V, initial pH of 5.0, and Cl− concentration of 0.1 mol/L, the removal efficiency was higher than 90.0 % after 1-h reaction when the berberine concentration was lower than 50 mg/L. The acute toxicity of the simulated berberine wastewater could be largely reduced under the optimum conditions. Ultraviolet–visible adsorption spectra and excitation–emission matrix fluorescence spectra also confirmed that although berberines could not be fully mineralized after 1 h of electrochemical treatment, most of them were easily oxidized and decomposed by the electrolysis, thus forming some intermediate products with simple aromatic structures. Based on the results obtained, the electrochemical process with Pt/Ti electrode can be a feasible alternative as a post-treatment or treatment for berberine wastewater.










Similar content being viewed by others
References
Baquero F (2001) Low-level antibacterial resistance: a gateway to clinical resistance. Drug Resist Update Rev comment Antimicrob Anticancer Chemother 4(2):93–105
Basha CA, Chithra E, Sripriyalakshmi NK (2009) Electro-degradation and biological oxidation of non-biodegradable organic contaminants. Chem Eng J 149(1–3):25–34
Bensalah N, Quiroz Alfaro MA, Martínez-Huitle CA (2009) Electrochemical treatment of synthetic wastewaters containing Alphazurine A dye. Chem Eng J 149(1–3):348–352
Bosco MV, Callao MP, Larrechi MS (2006) Simultaneous analysis of the photocatalytic degradation of polycyclic aromatic hydrocarbons using three-dimensional excitation–emission matrix fluorescence and parallel factor analysis. Anal Chim Acta 576(2):184–191
Bu HM, Wan J, Zhang Y, Meng W (2013) Spatial characteristics of surface water quality in the Haicheng River (Liao River basin) in Northeast China. Environ Earth Sci 70(6):2865–2872
Bundschuh M, Pierstorf R, Schreiber WH, Schulz R (2011) Positive effects of wastewater ozonation displayed by in situ bioassays in the receiving stream. Environ Sci Technol 45(8):3774–3780
Čerňáková M, Košťálová D (2002) Antimicrobial activity of berberine–a constituent of mahonia aquifolium. Folia Microbiol 47(4):375–378
Cilenti A, Provenzano MR, Senesi N (2005) Characterization of dissolved organic matter from saline soils by fluorescence spectroscopy. Environ Chem Lett 3(2):53–56
da Silva BF, Jelic A, Lopez-Serna R, Mozeto AA, Petrovic M, Barcelo D (2011) Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin Spain. Chemosphere 85(8):1331–1339
Daneshvar N, Khataee AR, Ghadim ARA, Rasoulifard MH (2007) Decolorization of CI Acid Yellow 23 solution by electrocoagulation process: investigation of operational parameters and evaluation of specific electrical energy consumption (SEEC). Journal Hazardous Materials 148(3):566–572
de Oliveira GR, Fernandes NS, de Melo JV, da Silva DR, Urgeghe C, Martínez-Huitle CA (2011) Electrocatalytic properties of Ti-supported Pt for decolorizing and removing dye from synthetic textile wastewaters. Chem Eng J 168(1):208–214
Drlica K (2003) The mutant selection window and antimicrobial resistance. J Antimicrob Chemother 52(1):11–17
Ellis JB (2006) Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ Pollut 144(1):184–189
Ghernaout D, Naceur MW, Aouabed A (2011) On the dependence of chlorine byproducts generated species formation of the electrode material and applied charge during electrochemical water treatment. Desalination 270(1–3):9–22
González T, Domínguez JR, Palo P, Sánchez-Martín J, Cuerda-Correa EM (2011) Development and optimization of the BDD-electrochemical oxidation of the antibiotic trimethoprim in aqueous solution. Desalination 280(1–3):197–202
Güven G, Perendeci A, Tanyolaç A (2009) Electrochemical treatment of simulated beet sugar factory wastewater. Chem Eng J 151(1–3):149–159
He PJ, Zheng Z, Zhang H, Shao LM, Tang QY (2009) PAEs and BPA removal in landfill leachate with Fenton process and its relationship with leachate DOM composition. Sci Total Environ 407(17):4928–4933
Heeb F, Singer H, Pernet-Coudrier B, Qi W, Liu H, Longree P, Mueller B, Berg M (2012) Organic micropollutants in rivers downstream of the megacity Bei**g: sources and mass fluxes in a large-scale wastewater irrigation system. Environ Sci Technol 46(16):8680–8688
Jara CC, Fino D, Specchia V, Saracco G, Spinelli P (2007) Electrochemical removal of antibiotics from wastewaters. Appl Catal B 70(1–4):479–487
Kosma CI, Lambropoulou DA, Albanis TA (2010) Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J Hazard Mater 179(1–3):804–817
Lahkimi A, Oturan MA, Oturan N, Chaouch M (2007) Removal of textile dyes from water by the electro-Fenton process. Environ Chem Lett 5(1):35–39
Li Y, Fu J, Deng SG, Lu XY (2014) Optimization of mesoporous carbons for efficient adsorption of berberine hydrochloride from aqueous solutions. J Colloid Interface Sci 424:104–112
Lin H, Niu JF, Xu JL, Huang HO, Li D, Yue ZH, Feng CH (2013a) Highly efficient and mild electrochemical mineralization of long-chain perfluorocarboxylic acids (C9–C10) by Ti/SnO2–Sb–Ce, Ti/SnO2–Sb/Ce–PbO2, and Ti/BDD Electrodes. Environ Sci Technol 47(22):13039–13046
Lin H, Niu JF, Xu JL, Li Y, Pan YH (2013b) Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: kinetics, reaction pathways, and energy cost evolution. Electrochim Acta 97:167–174
Martins RC, Rossi AF, Quinta-Ferreira RM (2010) Fenton’s oxidation process for phenolic wastewater remediation and biodegradability enhancement. J Hazard Mater 180(1–3):716–721
Michael I, Rizzo L, McArdell CS, Manaia CM, Merlin C, Schwartz T, Dagot C, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47(3):957–995
Niu JF, Lin H, Gong C, Sun XM (2013) Theoretical and experimental insights into the electrochemical mineralization mechanism of perfluorooctanoic acid. Environ Sci Technol 47(24):14341–14349
Qiu GL, Song YH, Zeng P, Duan L, **ao SH (2013) Characterization of bacterial communities in hybrid upflow anaerobic sludge blanket (UASB)-membrane bioreactor (MBR) process for berberine antibiotic wastewater treatment. Bioresour Technol 142:52–62
Ren MJ, Song YH, **ao SH, Zeng P, Peng JF (2011) Treatment of berberine hydrochloride wastewater by using pulse electro-coagulation process with Fe electrode. Chem Eng J 169(1–3):84–90
Rosal R, Rodriguez A, Perdigon-Melon JA, Petre A, Garcia-Calvo E, Gomez MJ, Aguera A, Fernandez-Alba AR (2010) Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res 44(2):578–588
Santos ID, Dezotti M, Dutra AJB (2013) Electrochemical treatment of effluents from petroleum industry using a Ti/RuO2 anode. Chem Eng J 226:293–299
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Bei**g China. Water Res 44(2):417–426
Sui Q, Huang J, Deng S, Chen W, Yu G (2011) Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ Sci Technol 45(8):3341–3348
Tavares MG, da Silva LVA, Solano AMS, Tonholo J, Martinez-Huitle CA, Zanta CLPS (2012) Electrochemical oxidation of Methyl Red using Ti/Ru0.3Ti0.7O2 and Ti/Pt anodes. Chem Eng J 204–206:141–150
Vanlangendonck Y, Corbisier D, Lierde AV (2005) Influence of operating conditions on the ammonia electro-oxidation rate in wastewaters from power plants. Water Res 39(19):3028–3034
Wu JJ, Yang JS, Muruganandham M, Wu CC (2008) The oxidation study of 2-propanol using ozone-based advanced oxidation processes. Sep Purif Technol 62(1):39–46
**ao SH, Qu JH, Zhao X, Liu HJ, Wan DJ (2009) Electrochemical process combined with UV light irradiation for synergistic degradation of ammonia in chloride-containing solutions. Water Res 43(5):1432–1440
**ng X, Zhu X, Li H, Jiang Y, Ni J (2012) Electrochemical oxidation of nitrogen heterocyclic compounds at boron-doped diamond electrode. Chemosphere 86:368–375
You G, Wang J (2011) Laboratory study of the electrochemical pre-oxidation for improving thermodynamic stability of an oilfield produced water. J Petrol Sci Eng 76(1–2):51–56
Zhang R, Tang J, Li J, Zheng Q, Liu D, Chen Y, Zou Y, Chen X, Luo C, Zhang G (2013) Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: occurrence, distribution and ecological risks. Environ Pollut 174:71–77
Acknowledgments
This work was supported by National Natural Science Foundation of China (NO. 21107103 and NO. 21277134) and China National Key Project of Science and Technology “Major Science and Technology Program for Water Pollution Control and Treatment” (2012ZX07202-002 and 2012ZX07202-005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tu, X., **ao, S., Song, Y. et al. Treatment of simulated berberine wastewater by electrochemical process with Pt/Ti anode. Environ Earth Sci 73, 4957–4966 (2015). https://doi.org/10.1007/s12665-015-4323-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4323-9