Abstract
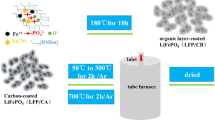
LiFePO4 was synthesized using hydrothermal method and coated with different amounts of citric acid as carbon source. The samples were characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscope (TEM), surface area measurement—Brunauer–Emmett–Teller (BET), discharge capability, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). The results show that the quality and thickness of the carbon coating on the surface of LiFePO4 particles are very important. The optimum carbon content (about 30 wt%) can lead to a more uniform carbon distribution. Electrochemical results show that the samples containing 20 wt%, 30 wt%, 40 wt%, and 50 wt% carbon deliver a discharge capacity of 105, 167, 151, and 112 mAh·g−1, respectively, at the rate of 0.1C. The increase of carbon content leads to the decrease of discharge capacity of LiFePO4/C, owing to the fact that excess carbon delays the diffusion of Li+ through the carbon layers during charge/discharge procedure. The LiFePO4/C with low carbon content exhibits poor electrochemical performance because of its low electrical conductivity. Therefore, the amount of carbon must be optimized in order to achieve excellent electrochemical performance of LiFePO4/C for its application in a lithium ion battery.







Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc. 1997;144(4):1188.
Liu J, Wang J, Yan X, Zhang X, Yang G, Jalbout AF, Wang R. Long-term cyclability of LiFePO4/carbon composite cathode material for lithium-ion battery applications. Electrochim Acta. 2009;54(24):5656.
Yamada A, Chung SC, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc. 2001;148(3):A224.
Kim J-K, Choi J-W, Chauhan GS, Ahn J-H, Hwang G-C, Choi J-B, Ahn H-J. Enhancement of electrochemical performance of lithium iron phosphate by controlled sol–gel synthesis. Electrochim Acta. 2008;53(28):8258.
Song Q, Ou X, Wang L, Liang G, Wang Z. Effect of pH value on particle morphology and electrochemical properties of LiFePO4 by hydrothermal method. Mater Res Bull. 2011;46(9):1398.
Yang S, Zavalij PY, Whittingham MS. Hydrothermal synthesis of lithium iron phosphate cathodes. Electrochem Commun. 2001;3(9):505.
Talebi-Esfandarani M, Savadogo O. Enhancement of electrochemical properties of platinum doped LiFePO4/C cathode material synthesized using hydrothermal method. Solid State Ion. 2014;261:81.
Ding Y, Jiang Y, Xu F, Yin J, Ren H, Zhuo Q, Long Z, Zhang P. Preparation of nano-structured LiFePO4/graphene composites by co-precipitation method. Electrochem Commun. 2010;12(1):10.
Deng FXZ, Zou J, Huang J, **ong X, Li X, Sheng H. Design and synthesis of in situ VGCFs improved LiFePO4 composite cathode materials. J New Mater Electrochem Syst. 2011;14(1):27.
Wang XY, Wu BR, Yang K, Yang XK, Wu C, Wang F, Wu F, Chen S, Li GW. Study on LiFePO4 material and the high power battery. J New Mater Electrochem Syst. 2009;12(4):213.
Franger SFLC, Bourbon C, Rouault H. LiFePO4 Synthesis routes for enhanced electrochemical performance. Electrochem Solid State Lett. 2002;5(10):A231.
Son J-T. High electrochemical performances of LiFePO4 cathode material prepared from surface modification by carbon coating using sucrose via sol-gel method. J New Mater Electrochem Syst. 2010;13(4):301.
Kuwahara A, Suzuki S, Miyayama M. Hydrothermal synthesis of LiFePO4 with small particle size and its electrochemical properties. J Electroceram. 2010;24(2):69.
Arumugam D, Kalaignan GP, Manisankar P. Synthesis and electrochemical characterizations of nano-crystalline LiFePO4 and Mg-doped LiFePO4 cathode materials for rechargeable lithium-ion batteries. J Solid State Electrochem. 2009;13(2):301.
Ou XQ, Liang GC, Liang JS, Xu SZ, Zhao X. LiFePO4 doped with magnesium prepared by hydrothermal reaction in glucose solution. Chin Chem Lett. 2008;19(3):345.
Zhang WJ. Comparison of the rate capacities of LiFePO4 cathode materials. J Electrochem Soc. 2010;157(10):A1040.
Devaraju MK, Honma I. Hydrothermal and solvothermal process towards development of LiMPO4 (M = Fe, Mn) nanomaterials for lithium-ion batteries. Adv Energy Mater. 2012;2(3):284.
Wang G, Liu H, Liu J, Qiao S, Lu GM, Munroe P, Ahn H. Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv Mater. 2010;22(44):4944.
Chernova NA, Roppolo M, Dillon AC, Whittingham MS. Layered vanadium and molybdenum oxides: batteries and electrochromics. J Mater Chem. 2009;19(17):2526.
Chen J, Vacchio MJ, Wang S, Chernova N, Zavalij PY, Whittingham MS. The hydrothermal synthesis and characterization of olivines and related compounds for electrochemical applications. Solid State Ion. 2008;178(31–32):1676.
Zaghib K, Mauger A, Gendron F, Julien CM. Surface effects on the physical and electrochemical properties of thin LiFePO4 particles. Chem Mater. 2007;20(2):462.
Yang J, Bai Y, Qing C, Zhang W. Electrochemical performances of Co-doped LiFePO4/C obtained by hydrothermal method. J Alloys Compd. 2011;509(37):9010.
Liang G, Wang L, Ou X, Zhao X, Xu S. Lithium iron phosphate with high-rate capability synthesized through hydrothermal reaction in glucose solution. J Power Sources. 2008;184(2):538.
Wang G, Shen X, Yao J. One-dimensional nanostructures as electrode materials for lithium-ion batteries with improved electrochemical performance. J Power Sources. 2009;189(1):543.
Zhou X, Wang F, Zhu Y, Liu Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries. J Mater Chem. 2011;21(10):3353.
Saravanan K, Balaya P, Reddy MV, Chowdari BVR, Vittal JJ. Morphology controlled synthesis of LiFePO4/C nanoplates for Li-ion batteries. Energy Environ Sci. 2010;3(4):457.
Su FY, You C, He YB, Lv W, Cui W, ** F, Li B, Yang QH, Kang F. Flexible and planar graphene conductive additives for lithium-ion batteries. J Mater Chem. 2010;20(43):9644.
Herle PS, Ellis B, Coombs N, Nazar LF. Nano-network electronic conduction in iron and nickel olivine phosphates. Nat Mater. 2004;3:147.
Doeff M, Wilcox J, Yu R, Aumentado A, Marcinek M, Kostecki R. Impact of carbon structure and morphology on the electrochemical performance of LiFePO4/C composites. J Solid State Electrochem. 2008;12(7):995.
Dominko R, Bele M, Gaberscek M, Remskar M, Hanzel D, Pejovnik S, Jamnik J. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites. J Electrochem Soc. 2005;152(3):A607.
Kang W, Zhao C, Liu R, Xu F, Shen Q. Ethylene glycol-assisted nanocrystallization of LiFePO4 for a rechargeable lithium-ion battery cathode. Cryst Eng Comm. 2012;14(6):2245.
Wang L, Wang H, Liu Z, **ao C, Dong S, Han P, Zhang Z, Zhang X, Bi C, Cui G. A facile method of preparing mixed conducting LiFePO4/graphene composites for lithium-ion batteries. Solid State Ion. 2010;181(37–38):1685.
Levi MD, Salitra G, Markovsky B, Teller H, Aurbach D, Heider U, Heider L. Solid-state electrochemical kinetics of Li-ion intercalation into Li1−x CoO2: simultaneous application of electroanalytical techniques SSCV, PITT, and EIS. J Electrochem Soc. 1999;146(4):1279.
Shenouda AY, Liu HK. Electrochemical behaviour of tin borophosphate negative electrodes for energy storage systems. J Power Sources. 2008;185(2):1386.
Ni J, Morishita M, Kawabe Y, Watada M, Takeichi N, Sakai T. Hydrothermal preparation of LiFePO4 nanocrystals mediated by organic acid. J Power Sources. 2010;195(9):2877.
Jiang Z, Jiang ZJ. Effects of carbon content on the electrochemical performance of LiFePO4/C core/shell nanocomposites fabricated using FePO4/polyaniline as an iron source. J Alloys Compd. 2012;537:308.
Acknowledgments
This work was financially supported by Laboratory of New Materials for Electrochemistry and Energy (LaNoMat), Polytechnique of Montreal, Quebec, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talebi-Esfandarani, M., Savadogo, O. Improvement of electrochemical and electrical properties of LiFePO4 coated with citric acid. Rare Met. 35, 303–308 (2016). https://doi.org/10.1007/s12598-015-0449-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0449-x