Abstract
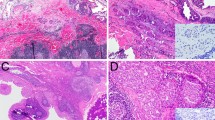
Intraductal carcinoma (IDC) is a rare salivary gland tumor that is considered analogous to ductal carcinoma in-situ of the breast, demonstrating a complex neoplastic epithelial proliferation surrounded by a continuous layer of presumed non-neoplastic myoepithelial cells. It is subcategorized into intercalated duct, apocrine, and hybrid subtypes based on morphologic and immunohistochemical features, with frequent NCOA4-RET and TRIM27-RET fusions, respectively, seen in intercalated duct and hybrid tumors. However, as an expanding clinicopathologic spectrum of IDC has been documented, controversy has emerged as to whether this tumor type is best defined by its intraductal growth pattern or distinctive molecular and immunophenotypic differentiation. Here, we further explore the nature of IDC by evaluating four cases that arose within intraparotid lymph nodes. These intercalated-duct phenotype tumors with diffuse S100 protein expression demonstrated a crowded and complex epithelial proliferation arranged in cystic, cribriform, and micropapillary architecture, surrounded by an intact myoepithelial cell layer, and were completely intranodal. Of two tumors with tissue available for molecular analysis, one demonstrated a NCOA4-RET fusion and one harbored a STRN-ALK fusion that is novel to IDC. Not only does the intranodal presence of IDC present a challenging differential diagnosis, but the complex nature of this proliferation within lymph node tissue raises questions as to whether the myoepithelial component of IDC is actually non-neoplastic in nature. Furthermore, identification of a STRN-ALK fusion expands the genetic spectrum of IDC and adds to evidence of an emerging role for ALK in salivary gland tumors. Further attention to the nature of the myoepithelial cells and documentation of alternate fusion events in IDC may inform continued discussion about its appropriate classification.


Similar content being viewed by others
References
Brandwein-Gensler M, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. World Health Organization classification of tumours. Lyon: IARC; 2005. p. 430.
Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28(8):1040–4.
Chen KT. Intraductal carcinoma of the minor salivary gland. J Laryngol Otol. 1983;97(2):189–91.
Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78(5):958–67.
Loening T, Leivo I, Simpson RHW, Weinreb I. Intraductal carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. p. 170–171.
Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53(4):416–25.
Skalova A, Ptakova N, Santana T, Agaimy A, Ihrler S, Uro-Coste E, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is "intraductal" correct? Am J Surg Pathol. 2019;43(10):1303–13.
Skalova A, Vanecek T, Uro-Coste E, Bishop JA, Weinreb I, Thompson LDR, et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol. 2018;42(11):1445–55.
Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, et al. Recurrent RET Gene Rearrangements in Intraductal Carcinomas of Salivary Gland. Am J Surg Pathol. 2018;42(4):442–52.
Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordonez B. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30(8):1014–21.
Bishop JA, Gagan J, Krane JF, Jo VY. Low-grade apocrine intraductal carcinoma: expanding the morphologic and molecular spectrum of an enigmatic salivary gland tumor. Head Neck Pathol. 2020. https://doi.org/10.1007/s12105-020-01128-0.
Guilmette J, Dias-Santagata D, Nose V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019;83:50–8.
Hsieh MS, Lee YH, ** YT, Kuo YJ. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch. 2020. https://doi.org/10.1007/s00428-020-02823-7.
Lu H, Graham RP, Seethala R, Chute D. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2020;14(1):239–45.
Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22(18):4623–33.
Dogan S, Ng CKY, Xu B, Kumar R, Wang L, Edelweiss M, et al. The repertoire of genetic alterations in salivary duct carcinoma including a novel HNRNPH3-ALK rearrangement. Hum Pathol. 2019;88:66–77.
Luk PP, Weston JD, Yu B, Selinger CI, Ekmejian R, Eviston TJ, et al. Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(Suppl 1):E1838–E18471847.
Lin SC, Ko RT, Kang BH, Wang JS. Intraductal carcinoma of salivary gland originating from an intraparotid lymph node: a case report. Malays J Pathol. 2019;41(2):207–11.
Weinreb I. Intraductal carcinoma of salivary gland (so-called low-grade cribriform cystadenocarcinoma) arising in an intraparotid lymph node. Head Neck Pathol. 2011;5(3):321–5.
Barakat N, Salman S, Nassar VH. Mucoepidermoid carcinoma in a lymph node of the parotid sheath simulating condylar tumor. Int J Oral Surg. 1973;2(1):26–30.
Minic AJ. Acinic cell carcinoma arising in a parotid lymph node. Int J Oral Maxillofac Surg. 1993;22(5):289–91.
Perzin KH, Livolsi VA. Acinic cell carcinoma arising in ectopic salivary gland tissue. Cancer. 1980;45(5):967–72.
Smith A, Winkler B, Perzin KH, Wazen J, Blitzer A. Mucoepidermoid carcinoma arising in an intraparotid lymph node. Cancer. 1985;55(2):400–3.
Wedell B, Burian P, Dahlenfors R, Stenman G, Mark J. Cytogenetical observations in a mucoepidermoid carcinoma arising from heterotopic intranodal salivary gland tissue. Oncol Rep. 1997;4(3):515–6.
Bishop JA, Gagan J, Baumhoer D, McLean-Holden AL, Oliai BR, Couce M, et al. Sclerosing polycystic "adenosis" of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2019. https://doi.org/10.1007/s12105-019-01088-0.
Haas BJ, Dobin A, Li B, Stransky N, Pochet N, Regev A. Accuracy assessment of fusion transcript detection via read-map** and de novo fusion transcript assembly-based methods. Genome Biol. 2019;20(1):213.
Kurian EM, Miller R, McLean-Holden AL, Oliai BR, Bishop JA. Low Molecular weight cytokeratin immunostaining for extrafollicular reticulum cells is an effective means of separating salivary gland tumor-associated lymphoid proliferation from true lymph node involvement. Head Neck Pathol. 2019. https://doi.org/10.1007/s12105-019-01080-8.
Nishijima T, Yamamoto H, Nakano T, Hatanaka Y, Taguchi KI, Masuda M, et al. Low-grade intraductal carcinoma (low-grade cribriform cystadenocarcinoma) with tumor-associated lymphoid proliferation of parotid gland. Pathol Res Pract. 2017;213(6):706–9.
Shinohara M, Harada T, Nakamura S, Oka M, Tashiro H. Heterotopic salivary gland tissue in lymph nodes of the cervical region. Int J Oral Maxillofac Surg. 1992;21(3):166–71.
Teymoortash A. Back to the roots of Warthin's tumor of the parotid gland. Eur Arch Otorhinolaryngol. 2013;270(9):2397–402.
Weiler C, Agaimy A, Zengel P, Zenk J, Kirchner T, Ihrler S. Nonsebaceous lymphadenoma of salivary glands: proposed development from intraparotid lymph nodes and risk of misdiagnosis. Virchows Arch. 2012;460(5):467–72.
Bastos AU, de Jesus AC, Cerutti JM. ETV6-NTRK3 and STRN-ALK kinase fusions are recurrent events in papillary thyroid cancer of adult population. Eur J Endocrinol. 2018;178(1):83–91.
Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci USA. 2014;111(11):4233–8.
Panebianco F, Nikitski AV, Nikiforova MN, Kaya C, Yip L, Condello V, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr Relat Cancer. 2019;26(11):803–14.
Perot G, Soubeyran I, Ribeiro A, Bonhomme B, Savagner F, Boutet-Bouzamondo N, et al. Identification of a recurrent STRN/ALK fusion in thyroid carcinomas. PLoS ONE. 2014;9(1):e87170.
Basturk O, Berger MF, Yamaguchi H, Adsay V, Askan G, Bhanot UK, et al. Pancreatic intraductal tubulopapillary neoplasm is genetically distinct from intraductal papillary mucinous neoplasm and ductal adenocarcinoma. Mod Pathol. 2017;30(12):1760–72.
Kusano H, Togashi Y, Akiba J, Moriya F, Baba K, Matsuzaki N, et al. Two cases of renal cell carcinoma harboring a novel STRN-ALK fusion gene. Am J Surg Pathol. 2016;40(6):761–9.
Yakirevich E, Resnick MB, Mangray S, Wheeler M, Jackson CL, Lombardo KA, et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin Cancer Res. 2016;22(15):3831–40.
Yang Y, Qin SK, Zhu J, Wang R, Li YM, **e ZY, et al. A rare STRN-ALK fusion in lung adenocarcinoma identified using next-generation sequencing-based circulating tumor DNA profiling exhibits excellent response to crizotinib. Mayo Clin Proc Innov Qual Outcomes. 2017;1(1):111–6.
Nakanishi Y, Masuda S, Iida Y, Takahashi N, Hashimoto S. Case report of non-small cell lung cancer with STRN-ALK translocation: a nonresponder to alectinib. J Thorac Oncol. 2017;12(12):e202–e204204.
Sasaki E, Masago K, Fujita S, Suzuki H, Hanai N, Hosoda W. Salivary secretory carcinoma harboring a novel ALK fusion: expanding the molecular characterization of carcinomas beyond the ETV6 gene. Am J Surg Pathol. 2020;44:962–9.
Funding
This study was funded in part by the Jane B. and Edwin P. Jenevein, MD Endowment for Pathology at UT Southwestern Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rooper, L.M., Thompson, L.D.R., Gagan, J. et al. Salivary Intraductal Carcinoma Arising within Intraparotid Lymph Node: A Report of 4 Cases with Identification of a Novel STRN-ALK Fusion. Head and Neck Pathol 15, 179–185 (2021). https://doi.org/10.1007/s12105-020-01198-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01198-0