Abstract
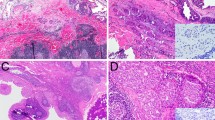
Intraductal carcinoma (IDC) is the current designation for a salivary gland neoplasm previously referred to as “low-grade salivary duct carcinoma” and “low-grade cribriform cystadenocarcinoma,” among others. IDC is conceptually believed to be similar to ductal carcinoma in-situ of the breast. Although IDC is one entity in the current WHO Classification of Head and Neck Tumors, recent studies have suggested that at least three subtypes exist: a low-grade, intercalated duct-like variant with frequent RET rearrangements; a high-grade apocrine variant with complex, salivary duct carcinoma-like genetics; and a mixed variant. We sought to characterize an unusual form of low-grade, purely apocrine IDC. Three cases of apocrine-type IDC with low-grade histology were retrieved from the authors’ consultation files. Immunohistochemistry for androgen receptor, GCDFP-15, S100, smooth muscle actin, and p40 was performed. A custom, targeted next generation sequencing (NGS) panel including 1425 cancer‐related genes was also done on all cases. All three cases developed in the parotid glands of men, aged 51, 63, and 73 years (mean, 62 years). All cases consisted of large, rounded macrocysts surrounded by smaller nests which were lined by cells with abundant granular eosinophilic cytoplasm and large round nuclei with prominent nucleoli. Pleomorphism was mild, the mitotic rate was low, and necrosis was absent. No cases had any invasive foci or areas of intercalated duct-like morphology. By immunohistochemistry, all cases were diffusely positive for androgen receptor and GCDFP-15, surrounded entirely by an intact layer of small myoepithelial cells positive for S100, smooth muscle actin, and p40. Targeted NGS results were obtained from two cases: both harbored HRAS mutations and copy number losses in TP53, while one case each harbored mutations in PIK3CA, SPEN, and ATM. Fusions were absent in both cases. All three patients were treated by surgery alone, and are currently free of disease (follow up 12–190 months). This study confirms the existence of a low-grade, purely apocrine form of IDC. In its pure form, i.e., without an intercalated duct-type component, low-grade apocrine IDC is genetically similar to high-grade salivary duct carcinoma, with frequent HRAS and PI3K pathway mutations. Despite its molecular similarities to the aggressive salivary duct carcinoma, low-grade apocrine IDC appears to behave in a very indolent manner, supporting is classification as a non-invasive neoplasm, and underscoring the need to distinguish these tumors from each other.



Similar content being viewed by others
References
Loening T, Leivo I, Simpson RHW, et al. Intraductal carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO Classification of Head and Neck Tumours. Lyon, France: IARC Press; 2017. p. 170–1.
Brandwein-Gensler M, Hille J, Wang BY, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–4.
Bishop JA, Gagan J, Baumhoer D, et al. Sclerosing polycystic “adenosis” of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2019.
Skalova A, Vanecek T, Simpson RHW, et al. Molecular advances in salivary gland pathology and their practical application. Diagn Histopathol. 2012;18:388–96.
Chen KT. Intraductal carcinoma of the minor salivary gland. J Laryngol Otol. 1983;97:189–91.
Anderson C, Muller R, Piorkowski R, et al. Intraductal carcinoma of major salivary gland. Cancer. 1992;69:609–14.
Tatemoto Y, Ohno A, Osaki T. Low malignant intraductal carcinoma on the hard palate: a variant of salivary duct carcinoma? Eur J Cancer B Oral Oncol. 1996;32B:275–7.
Watatani K, Shirasuna K, Aikawa T, et al. Intraductal carcinoma of the tongue: report of a case. Int J Oral Maxillofac Surg. 1991;20:175–6.
Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma: a distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–67.
Brandwein-Gensler MS, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumors. Lyon, France: IARC Press; 2005. p. 233.
Weinreb I, Tabanda-Lichauco R, Van der Kwast T, et al. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30:1014–21.
Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53:416–25.
Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39:705–13.
Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288–94.
Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–33.
Ku BM, Jung HA, Sun JM, et al. High-throughput profiling identifies clinically actionable mutations in salivary duct carcinoma. J Transl Med. 2014;12:299.
Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(Suppl 1):E1838–47.
Jaehne M, Roeser K, Jaekel T, et al. Clinical and immunohistologic ty** of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526–33.
Weinreb I, Bishop JA, Chiosea SI, et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am J Surg Pathol. 2018;42:442–52.
Skalova A, Vanecek T, Uro-Coste E, et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol. 2018;42:1445–55.
Skalova A, Ptakova N, Santana T, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is "intraductal" correct? Am J Surg Pathol. 2019;43:1303–13.
Lu H, Graham RP, Seethala R, et al. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2019.
Nakaguro M, Urano M, Suzuki H, et al. Low-grade intraductal carcinoma of the salivary gland with prominent oncocytic change: a newly described variant. Histopathology. 2018;73:314–20.
Canas Marques R, Felix A. Invasive carcinoma arising from sclerosing polycystic adenosis of the salivary gland. Virchows Arch. 2014;464:621–5.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB 112017-073), which did not require informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bishop, J.A., Gagan, J., Krane, J.F. et al. Low-grade Apocrine Intraductal Carcinoma: Expanding the Morphologic and Molecular Spectrum of an Enigmatic Salivary Gland Tumor. Head and Neck Pathol 14, 869–875 (2020). https://doi.org/10.1007/s12105-020-01128-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01128-0