Abstract
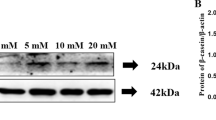
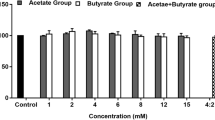
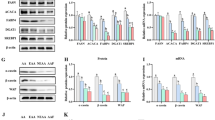
Although conjugated linoleic acid (CLA) can promote human health, its content in milk is insufficient to have a significant impact. The majority of the CLA in milk is produced endogenously by the mammary gland. However, research on improving its content through nutrient-induced endogenous synthesis is relatively scarce. Previous research found that the key enzyme, stearoyl-CoA desaturase (SCD) for the synthesis of CLA, can be expressed more actively in bovine mammary epithelial cells (MAC-T) when lithium chloride (LiCl) is present. This study investigated whether LiCl can encourage CLA synthesis in MAC-T cells. The results showed that LiCl effectively increased SCD and proteasome α5 subunit (PSMA5) protein expression in MAC-T cells as well as the content of CLA and its endogenous synthesis index. LiCl enhanced the expression of proliferator-activated receptor-γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), and its downstream enzymes acetyl CoA carboxylase (ACC), fatty acid synthase (FASN), lipoprotein lipase (LPL), and Perilipin 2 (PLIN2). The addition of LiCl significantly enhanced p-GSK-3β, β-catenin, p-β-catenin protein expression, hypoxia-inducible factor-1α (HIF-1α), and downregulation factor genes for mRNA expression (P < 0.05). These findings highlight that LiCl can increase the expression of SCD and PSMA5 by activating the transcription of HIF-1α, Wnt/β-catenin, and the SREBP1 signaling pathways to promote the conversion of trans-vaccenic acid (TVA) to the endogenous synthesis of CLA. This data suggests that the exogenous addition of nutrients can increase CLA content in milk through pertinent signaling pathways.






Similar content being viewed by others
Data Availability
Data are available for consultation upon request to the corresponding author.
References
den Hartigh LJ (2019) Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients 11. https://doi.org/10.3390/nu11020370
Domagala D, Leszczynska T, Koronowicz A, Domagala B, Drozdowska M, Piasna-Slupecka E (2021) Mechanisms of anticancer activity of a fatty acid mixture extracted from hen egg yolks enriched in conjugated linoleic acid diene (CLA) against WM793 Melanoma Cells. Nutrients 13. https://doi.org/10.3390/nu13072348
Fujita Y, Kano K, Kishino S, Nagao T, Shen XF, Sato C, Hatakeyama H, Ota Y, Niibori S, Nomura A et al (2021) Dietary cis-9, trans-11-conjugated linoleic acid reduces amyloid beta-protein accumulation and upregulates anti-inflammatory cytokines in an Alzheimer’s disease mouse model. Sci Rep 11(1):9749. https://doi.org/10.1038/s41598-021-88870-9
Griinari JM, Corl BA, Lacy SH, Chouinard PY, Nurmela KVV, Bauman DE (2000) Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta(9)-desaturase. J Nutr 130:2285–2291. https://doi.org/10.1093/jn/130.9.2285
Kuhl GC, Mazzon RR, Simas Porto BL, Zamboni Madaloz T, Razzera G, Patricio DO, Linehan K, Ahern G, Mathur H, Ross P et al (2021) Oleate hydratase in Lactobacillus delbrueckii subsp. bulgaricus LBP UFSC 2230 catalyzes the reversible conversion between linoleic acid and ricinoleic acid. Microbiol Spectr 9:e0117921. https://doi.org/10.1128/Spectrum.01179-21
Toral PG, Hervas G, Frutos P (2022) Effect of lipid supplementation on the endogenous synthesis of milk cis-9, trans-11 conjugated linoleic acid in dairy sheep and goats: a tracer assay with (13)C-vaccenic acid. J Dairy Sci 105:255–268. https://doi.org/10.3168/jds.2021-20728
Song J, Wang YJ, Fan XQ, Wu HW, Han JH, Yang M, Lu L, Nie GH (2019) Trans-vaccenic acid inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells via a mitochondrial-mediated apoptosis pathway. Lipids Health Dis 18(1):46. https://doi.org/10.1186/s12944-019-0993-8
** YC, Lee HG, Xu CX, Han JA, Choi SH, Song MK, Kim YJ, Lee KB, Kim SK, Kang HS et al (2010) Proteomic analysis of endogenous conjugated linoleic acid biosynthesis in lactating rats and mouse mammary gland epithelia cells (HC11). Biochim Biophys Acta 1804:745–751. https://doi.org/10.1016/j.bbapap.2009.11.016
Wang T, Lee H, Zhen Y (2018) Responses of MAC-T cells to inhibited stearoyl-CoA desaturase 1 during cis-9, trans-11 conjugated linoleic acid synthesis. Lipids 53:647–652. https://doi.org/10.1002/lipd.12077
Rioux V, Legrand P (2019) Fatty acid desaturase 3 (FADS3) is a specific 13-desaturase of ruminant trans-vaccenic acid. Lifestyle Genom 12:18–24. https://doi.org/10.1159/000502356
Kikuchi K, Tsukamoto H (2020) Stearoyl-CoA desaturase and tumorigenesis. Chem Biol Interact 316:108917. https://doi.org/10.1016/j.cbi.2019.108917
** YC, Li ZH, Hong ZS, Xu CX, Han JA, Choi SH, Yin JL, Zhang QK, Lee KB, Kang SK et al (2012) Conjugated linoleic acid synthesis-related protein proteasome subunit alpha 5 (PSMA5) is increased by vaccenic acid treatment in goat mammary tissue. J Dairy Sci 95:4286–4297. https://doi.org/10.3168/jds.2011-4281
Liu X, Shen J, Zong J, Liu J, ** Y (2021) Beta-sitosterol promotes milk protein and fat syntheses-related genes in bovine mammary epithelial cells. Animals (Basel) 11. https://doi.org/10.3390/ani11113238
Zong J, Shen J, Liu X, Liu J, Zhang J, Zhou C, Fan Y, ** Y (2022) Lithium chloride promotes milk protein and fat synthesis in bovine mammary epithelial cells via HIF-1alpha and beta-catenin signaling pathways. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03131-8
Woodward TL, Turner JD, Hung HT, Zhao X (1996) Inhibition of cellular proliferation and modulation of insulin-like growth factor binding proteins by retinoids in a bovine mammary epithelial cell line. J Cell Physiol 167:488–499. https://doi.org/10.1002/(SICI)1097-4652(199606)167:3<488::AID-JCP13>3.0.CO;2-0
Huynh HT, Robitaille G, Turner JD (1991) Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp Cell Res 197:191–199. https://doi.org/10.1016/0014-4827(91)90422-q
Petit CA, Gardes M, Feunteun J (1983) Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology 127:74–82. https://doi.org/10.1016/0042-6822(83)90372-0
Ontsouka EC, Bertschi JS, Huang X, Luthi M, Muller S, Albrecht C (2016) Can widely used cell type markers predict the suitability of immortalized or primary mammary epithelial cell models? Biol Res 49:1. https://doi.org/10.1186/s40659-015-0063-2
Ogunnaike M, Wang H, Zempleni J (2021) Bovine mammary alveolar MAC-T cells afford a tool for studies of bovine milk exosomes in drug delivery. Int J Pharm 610:121263. https://doi.org/10.1016/j.ijpharm.2021.121263
Li L, Tang W, Zhao M, Gong B, Cao M, Li J (2021) Study on the regulation mechanism of lipopolysaccharide on oxidative stress and lipid metabolism of bovine mammary epithelial cells. Physiol Res 70:777–785. https://doi.org/10.33549/physiolres.934682
Wang X, Zhang M, Jiang N, Zhang A (2018) Sodium phenylbutyrate ameliorates inflammatory response induced by staphylococcus aureus lipoteichoic acid via suppressing TLR2/NF-kappaB/NLRP3 pathways in MAC-T cells. Molecules 23. https://doi.org/10.3390/molecules23123056
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Huebner SM, Olson JM, Campbell JP, Bishop JW, Crump PM, Cook ME (2016) Low dietary c9t11-conjugated linoleic acid intake from dairy fat or supplements reduces inflammation in collagen-induced arthritis. Lipids 51:807–819. https://doi.org/10.1007/s11745-016-4163-8
Schaftenaar F, Frodermann V, Kuiper J, Lutgens E (2016) Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol 27:209–215. https://doi.org/10.1097/MOL.0000000000000302
Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Phillips BS, Bauman DE (2001) The role of Delta(9)-desaturase in the production of cis-9, trans-11 CLA. J Nutr Biochem 12:622–630. https://doi.org/10.1016/S0955-2863(01)00180-2
Corl BA, Barbano DM, Bauman DE, Ip C (2003) cis-9, trans-11 CLA derived endogenously from trans-11 18:1 reduces cancer risk in rats. J Nutr 133:2893–2900. https://doi.org/10.1093/jn/133.9.2893
Lee YJ, Jenkins TC (2011) Biohydrogenation of linolenic acid to stearic acid by the rumen microbial population yields multiple intermediate conjugated diene isomers. J Nutr 141:1445–1450. https://doi.org/10.3945/jn.111.138396
Peng LY, Bai G, Wang CZ, Dong JN, Liu YJ, Sun Z, Zhen YG, Qin GX, Zhang XF, Demelash N et al (2022) Proteomics insights into the gene network of cis9, trans11-conjugated linoleic acid biosynthesis in bovine mammary gland epithelial cells. Animals-Basel 12(13):1718. https://doi.org/10.3390/ani12131718
Bernard L, Rouel J, Leroux C, Ferlay A, Faulconnier Y, Legrand P, Chilliard Y (2005) Mammary lipid metabolism and milk fatty acid secretion in alpine goats fed vegetable lipids. J Dairy Sci 88:1478–1489. https://doi.org/10.3168/jds.S0022-0302(05)72816-2
Conte G, Mele M, Chessa S, Castiglioni B, Serra A, Pagnacco G, Secchiari P (2010) Diacylglycerol acyltransferase 1, stearoyl-CoA desaturase 1, and sterol regulatory element binding protein 1 gene polymorphisms and milk fatty acid composition in Italian Brown cattle. J Dairy Sci 93:753–763. https://doi.org/10.3168/jds.2009-2581
Zhang JJ, Hao JJ, Zhang YR, Wang YL, Li MY, Miao HL, Zou XJ, Liang B (2017) Zinc mediates the SREBP-SCD axis to regulate lipid metabolism in Caenorhabditis elegans. J Lipid Res 58:1845–1854. https://doi.org/10.1194/jlr.M077198
Tudisco R, Morittu VM, Addi L, Moniello G, Grossi M, Musco N, Grazioli R, Mastellone V, Pero ME, Lombardi P et al (2019) Influence of pasture on stearoyl-CoA desaturase and miRNA 103 expression in goat milk: preliminary results. Animals (Basel) 9. https://doi.org/10.3390/ani9090606
Hu Y, Wang Y, Wang X, Wu X, Fu L, Liu X, Wen Y, Sheng J, Zhang J (2021) The role of cation diffusion facilitator CDF-1 in lipid metabolism in Caenorhabditis elegans. G3 (Bethesda). https://doi.org/10.1093/g3journal/jkab120
Oshino N, Sato R (1972) The dietary control of the microsomal stearyl CoA desaturation enzyme system in rat liver. Arch Biochem Biophys 149:369–377. https://doi.org/10.1016/0003-9861(72)90335-9
Kato H, Sakaki K, Mihara K (2006) Ubiquitin-proteasome-dependent degradation of mammalian ER stearoyl-CoA desaturase. J Cell Sci 119:2342–2353. https://doi.org/10.1242/jcs.02951
Wang T, Lee SB, Hwang JH, Lim JN, Jung US, Kim MJ, Kang HS, Choi SH, Lee JS, Roh SG et al (2015) Proteomic analysis reveals PGAM1 altering cis-9, trans-11 conjugated linoleic acid synthesis in bovine mammary gland. Lipids 50:469–481. https://doi.org/10.1007/s11745-015-4009-9
Lin X, Loor JJ, Herbein JH (2004) Trans10, cis12-18:2 is a more potent inhibitor of de novo fatty acid synthesis and desaturation than cis9, trans11-18:2 in the mammary gland of lactating mice. J Nutr 134:1362–1368. https://doi.org/10.1093/jn/134.6.1362
Lu F, Zhou J, Chen Q, Zhu J, Zheng X, Fang N, Qiao L (2022) PSMA5 contributes to progression of lung adenocarcinoma in association with the JAK/STAT pathway. Carcinogenesis. https://doi.org/10.1093/carcin/bgac046
Chiao CC, Liu YH, Phan NN, An Ton NT, Ta HDK, Anuraga G, Minh Xuan DT, Fitriani F, Putri Hermanto EM, Athoillah M et al (2021) Prognostic and genomic analysis of proteasome 20s subunit alpha (PSMA) family members in breast cancer. Diagnostics (Basel) 11. https://doi.org/10.3390/diagnostics11122220
Aumeistere L, Belusko A, Ciprovica I, Zavadska D (2021) Trans fatty acids in human milk in Latvia: association with dietary habits during the lactation period. Nutrients 13. https://doi.org/10.3390/nu13092967
Mojska H, Socha P, Socha J, Soplinska E, Jaroszewska-Balicka W, Szponar L (2003) Trans fatty acids in human milk in Poland and their association with breastfeeding mothers’ diets. Acta Paediatr 92:1381–1387. https://doi.org/10.1080/08035250310006692
Bionaz M, Loor JJ (2008) Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 9:366. 2008/08/02 Edition
Xu HF, Luo J, Zhao WS, Yang YC, Tian HB, Shi HB, Bionaz M (2016) Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J Dairy Sci 99:783–795. https://doi.org/10.3168/jds.2015-9736
Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A 100:12027–12032. https://doi.org/10.1073/pnas.1534923100
Li J, Luo J, Xu H, Wang M, Zhu J, Shi H, Haile AB, Wang H, Sun Y (2015) Fatty acid synthase promoter: characterization, and transcriptional regulation by sterol regulatory element binding protein-1 in goat mammary epithelial cells. Gene 561:157–164. https://doi.org/10.1016/j.gene.2015.02.034
Yao D, Luo J, He Q, Shi H, Li J, Wang H, Xu H, Chen Z, Yi Y, Loor JJ (2017) SCD1 alters long-chain fatty acid (LCFA) composition and its expression is directly regulated by SREBP-1 and PPARgamma 1 in Dairy Goat Mammary Cells. J Cell Physiol 232:635–649. https://doi.org/10.1002/jcp.25469
Zhu L, Du W, Liu Y, Cheng M, Wang X, Zhang C, Lv X, Li F, Zhao S, Hao J (2019) Prolonged high-glucose exposure decreased SREBP-1/FASN/ACC in Schwann cells of diabetic mice via blocking PI3K/Akt pathway. J Cell Biochem 120:5777–5789. https://doi.org/10.1002/jcb.27864
Aryal B, Price NL, Suarez Y, Fernandez-Hernando C (2019) ANGPTL4 in metabolic and cardiovascular disease. Trends Mol Med 25:723–734. https://doi.org/10.1016/j.molmed.2019.05.010
Hao Z, Luo Y, Wang J, Hickford JGH, Zhou H, Hu J, Liu X, Li S, Shen J, Ke N et al (2021) MicroRNA-432 inhibits milk fat synthesis by targeting SCD and LPL in ovine mammary epithelial cells. Food Funct 12:9432–9442. https://doi.org/10.1039/d1fo01260f
Auwerx J (1999) PPARgamma, the ultimate thrifty gene. Diabetologia 42:1033–1049. https://doi.org/10.1007/s001250051268
Janani C, Ranjitha Kumari BD (2015) PPAR gamma gene–a review. Diabetes Metab Syndr 9:46–50. https://doi.org/10.1016/j.dsx.2014.09.015
Meng FG, Zhang XN, Liu SX, Wang YR, Zeng T (2020) Roles of peroxisome proliferator-activated receptor alpha in the pathogenesis of ethanol-induced liver disease. Chem Biol Interact 327:109176. https://doi.org/10.1016/j.cbi.2020.109176
Khan D, Ara T, Ravi V, Rajagopal R, Tandon H, Parvathy J, Gonzalez EA, Asirvatham-Jeyaraj N, Krishna S, Mishra S et al (2021) SIRT6 transcriptionally regulates fatty acid transport by suppressing PPARgamma. Cell Rep 35:109190. https://doi.org/10.1016/j.celrep.2021.109190
Pawlak P, Malyszka N, Szczerbal I, Kolodziejski P (2020) Fatty acid induced lipolysis influences embryo development, gene expression and lipid droplet formation in the porcine cumulus cellsdagger. Biol Reprod 103:36–48. https://doi.org/10.1093/biolre/ioaa045
Lee Y, Kim SM, Jung EH, Park J, Lee JW, Han IO (2020) Lithium chloride promotes lipid accumulation through increased reactive oxygen species generation. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158552. https://doi.org/10.1016/j.bbalip.2019.158552
Chi YY, Shen JL, Zhang J, Shan AS, Niu SL, Zhou CH, Lee HG, ** YC (2016) Lithium chloride’s inhibition of 3T3-L1 cell differentiation by regulating the Wnt/beta-catenin pathway and enhancing villin 2 expression. Food Sci Biotechnol 25:1147–1153. https://doi.org/10.1007/s10068-016-0183-7
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. https://doi.org/10.1146/annurev.cellbio.20.010403.113126
Chin PC, Majdzadeh N, D’Mello SR (2005) Inhibition of GSK3 beta is a common event in neuroprotection by different survival factors. Mol Brain Res 137:193–201. https://doi.org/10.1016/j.molbrainres.2005.03.004
Undi RB, Gutti U, Gutti RK (2017) LiCl regulates mitochondrial biogenesis during megakaryocyte development. J Trace Elem Med Biol 39:193–201. https://doi.org/10.1016/j.jtemb.2016.10.003
Mirakhori F, Zeynali B, Tafreshi AP, Shirmohammadian A (2013) Lithium induces follicular atresia in rat ovary through a GSK-3beta/beta-catenin dependent mechanism. Mol Reprod Dev 80:286–296. https://doi.org/10.1002/mrd.22163
Bai L, Chang HM, Cheng JC, Chu G, Leung PCK, Yang G (2018) Lithium chloride inhibits StAR and progesterone production through GSK-3beta and ERK1/2 signaling pathways in human granulosa-lutein cells. Mol Cell Endocrinol 461:89–99. https://doi.org/10.1016/j.mce.2017.08.018
Liu X, Lu X, Song K, Blackman MR (2016) Natural functions of PLIN2 mediating Wnt/LiCl signaling and glycogen synthase kinase 3 (GSK3)/GSK3 substrate-related effects are modulated by lipid. Mol Cell Biol 36:421–437. https://doi.org/10.1128/MCB.00510-15
Xu H, Wang J, Zhang X, Li Z, Wang Y, Xue C (2015) Inhibitory effect of fucosylated chondroitin sulfate from the sea cucumber Acaudina molpadioides on adipogenesis is dependent on Wnt/beta-catenin pathway. J Biosci Bioeng 119:85–91. https://doi.org/10.1016/j.jbiosc.2014.05.026
Abiola M, Favier M, Christodoulou-Vafeiadou E, Pichard AL, Martelly I, Guillet-Deniau I (2009) Activation of Wnt/beta-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. Plos One 4:e8509. https://doi.org/10.1371/journal.pone.0008509
Shao Y, Zhao FQ (2014) Emerging evidence of the physiological role of hypoxia in mammary development and lactation. J Anim Sci Biotechnol 5:9. https://doi.org/10.1186/2049-1891-5-9
Badowska-Kozakiewicz AM, Sobol M, Patera J (2017) Expression of multidrug resistance protein P-glycoprotein in correlation with markers of hypoxia (HIF-1alpha, EPO, EPO-R) in invasive breast cancer with metastasis to lymph nodes. Arch Med Sci 13:1303–1314. https://doi.org/10.5114/aoms.2016.62723
Penna F, Busquets S, Toledo M, Pin F, Massa D, Lopez-Soriano FJ, Costelli P, Argiles JM (2013) Erythropoietin administration partially prevents adipose tissue loss in experimental cancer cachexia models. J Lipid Res 54:3045–3051. https://doi.org/10.1194/jlr.M038406
Choi K, ** M, Zouboulis CC, Lee Y (2021) Increased lipid accumulation under hypoxia in SZ95 human sebocytes. Dermatology 237:131–141. https://doi.org/10.1159/000505537
Baumeister W, Walz J, Zuhl F, Seemuller E (1998) The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367–380. https://doi.org/10.1016/s0092-8674(00)80929-0
Voges D, Zwickl P, Baumeister W (1999) The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68:1015–1068. https://doi.org/10.1146/annurev.biochem.68.1.1015
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428. https://doi.org/10.1152/physrev.00027.2001
Tanaka K (2009) The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci 85:12–36. https://doi.org/10.2183/pjab.85.12
Collins GA, Goldberg AL (2017) The logic of the 26S proteasome. Cell 169:792–806. https://doi.org/10.1016/j.cell.2017.04.023
Fu Z, Lu C, Zhang C, Qiao B (2019) PSMA5 promotes the tumorigenic process of prostate cancer and is related to bortezomib resistance. Anticancer Drugs 30:e0773. https://doi.org/10.1097/CAD.0000000000000773
Livnat-Levanon N, Glickman MH (2011) Ubiquitin-proteasome system and mitochondria - reciprocity. Biochim Biophys Acta 1809:80–87. https://doi.org/10.1016/j.bbagrm.2010.07.005
Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM (2007) Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171:513–524. https://doi.org/10.2353/ajpath.2007.070188
Sulkshane P, Ram J, Thakur A, Reis N, Kleifeld O, Glickman MH (2021) Ubiquitination and receptor-mediated mitophagy converge to eliminate oxidation-damaged mitochondria during hypoxia. Redox Biol 45:102047. https://doi.org/10.1016/j.redox.2021.102047
An T, Gong Y, Li X, Kong L, Ma P, Gong L, Zhu H, Yu C, Liu J, Zhou H et al (2017) USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol 131:29–39. https://doi.org/10.1016/j.bcp.2017.02.011
Glaeser K, Urban M, Fenech E, Voloshanenko O, Kranz D, Lari F, Christianson JC, Boutros M (2018) ERAD-dependent control of the Wnt secretory factor Evi. EMBO J 37. https://doi.org/10.15252/embj.201797311
Voutsadakis IA (2010) Peroxisome proliferator activated receptor-gamma and the ubiquitin-proteasome system in colorectal cancer. World J Gastrointest Oncol 2:235–241. https://doi.org/10.4251/wjgo.v2.i5.235
Wang DT, Lu L, Shi Y, Geng ZB, Yin Y, Wang M, Wei LB (2014) Supplementation of ketoacids contributes to the up-regulation of the Wnt7a/Akt/p70S6K pathway and the down-regulation of apoptotic and ubiquitin-proteasome systems in the muscle of 5/6 nephrectomised rats. Br J Nutr 111:1536–1548. https://doi.org/10.1017/S0007114513004091
Acknowledgements
We gratefully acknowledge Professor Hong-Gu Lee (Department of Animal Science and Technology, Sanghuh College of Life Sciences, Konkuk University, Seoul 05029, Korea) for generously provided MAC-T cells for cell culture assays.
Funding
This study was funded by the Jilin Provincial Department of Education (JJKH20201022KJ) and the National Natural Science Foundation of China (31301996).
Author information
Authors and Affiliations
Contributions
Conceptualization, Yongcheng ** and **glin Shen; methodology, **xin Zong; validation, **xin Zong, and Jiayi Liu; investigation, Yating Fan and Junhao Cui; resources, **glin Shen.; data curation, **xin Zong; writing—original draft preparation, Jiayi Liu; writing—review and editing, Yongcheng ** and Dongqiao Peng; visualization, Jiayi Liu; supervision, Yongcheng **,**glin Shen and Dongqiao Peng; project administration, Yongcheng **; funding acquisition, Yongcheng **. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study does not involve any human or animal testing.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Shen, J., Zong, J. et al. Lithium Chloride Promotes Endogenous Synthesis of CLA in Bovine Mammary Epithelial Cells. Biol Trace Elem Res 202, 513–526 (2024). https://doi.org/10.1007/s12011-023-03679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03679-z