Abstract
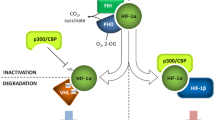
Hypoxia is a physiological or pathological condition of a deficiency of oxygen supply in the body as a whole or within a tissue. During hypoxia, tissues undergo a series of physiological responses to defend themselves against a low oxygen supply, including increased angiogenesis, erythropoiesis, and glucose uptake. The effects of hypoxia are mainly mediated by hypoxia-inducible factor 1 (HIF-1), which is a heterodimeric transcription factor consisting of α and β subunits. HIF-1β is constantly expressed, whereas HIF-1α is degraded under normal oxygen conditions. Hypoxia stabilizes HIF-1α and the HIF complex, and HIF then translocates into the nucleus to initiate the expression of target genes. Hypoxia has been extensively studied for its role in promoting tumor progression, and emerging evidence also indicates that hypoxia may play important roles in physiological processes, including mammary development and lactation. The mammary gland exhibits an increasing metabolic rate from pregnancy to lactation to support mammary growth, lactogenesis, and lactation. This process requires increasing amounts of oxygen consumption and results in localized chronic hypoxia as confirmed by the binding of the hypoxia marker pimonidazole HCl in mouse mammary gland. We hypothesized that this hypoxic condition promotes mammary development and lactation, a hypothesis that is supported by the following several lines of evidence: i) Mice with an HIF-1α deletion selective for the mammary gland have impaired mammary differentiation and lipid secretion, resulting in lactation failure and striking changes in milk compositions; ii) We recently observed that hypoxia significantly induces HIF-1α-dependent glucose uptake and GLUT1 expression in mammary epithelial cells, which may be responsible for the dramatic increases in glucose uptake and GLUT1 expression in the mammary gland during the transition period from late pregnancy to early lactation; and iii) Hypoxia and HIF-1α increase the phosphorylation of signal transducers and activators of transcription 5a (STAT5a) in mammary epithelial cells, whereas STAT5 phosphorylation plays important roles in the regulation of milk protein gene expression and mammary development. Based on these observations, hypoxia effects emerge as a new frontier for studying the regulation of mammary development and lactation.
Similar content being viewed by others
Introduction
Oxygen is critical for cellular aerobic metabolism in many higher organisms, including mammals, as it is the final electron acceptor in the electron transport chain of oxidative phosphorylation in mitochondria (Figure 1). Aerobic metabolism is 19 times more efficient in energy production than anaerobic metabolism: a molecule of glucose, the major energy source in most mammalian cells, produces up to 38 molecules of ATP in aerobic metabolism though only 2 in anaerobic metabolism [1]. In addition to efficient energy production, aerobic metabolism produces the end product H2O, whereas anaerobic metabolism produces lactate, which is normally removed in the liver with the requirement of oxygen in mammals. Therefore, an oxygen supply and the maintenance of oxygen homeostasis are essential in aerobic organisms.
Effects of hypoxia on glucose metabolism in cells. Glucose, taken up by facilitative glucose transporter 1 (GLUT1), is first phosphorylated to glucose-6-phosphate by hexokinase (HK) and is then converted to pyruvate by glycolytic enzymes. Lactate dehydrogenase A (LDHA) converts pyruvate to lactate when oxygen is limited. In well-oxygenated cells, pyruvate is actively taken up by the mitochondria and converted to acetyl coenzyme A (CoA) by pyruvate dehydrogenase complex (PDC), which can be inactivated via phosphorylation by pyruvate dehydrogenase kinase 1 (PDK1). Acetyl-CoA enters the tricarboxylic acid cycle (TCA) to produce NADH, which is used to produce ATP through the electron transport chain (ETC) via the transfer of electrons to oxygen to form water. The major enzymes affected by hypoxia are indicated. “+” = stimulation.
Tissues in the body are exposed to different levels of oxygen, which are affected by the oxygen supply and tissue metabolic rate and range from near 159 mm Hg in the upper airway [the O2 partial pressure in the atmosphere at sea level (pO2); 21% of atmospheric air] to as low as 5 mm Hg (~1%) in the retina [2]. In most tissues, the oxygen tension is between 10 and 45 mm Hg (3-6%) [3, 4]. Hypoxia is a deficiency of oxygen supply in the entire body (such as in high elevation exposure) or locally within a tissue when the oxygen delivery or availability is reduced due to different causes (such as tissue injury). The precise hypoxic pO2 is different between organs, but a venous pO2 of below 6% O2 can induce a hypoxic response in most tissues, and 0.5-1% induces the maximal effects [5].
The body and its tissues have specific mechanisms to sense oxygen levels and make the necessary adaptations for survival under hypoxic conditions. Global oxygen is sensed by central chemoreceptors located on ventrolateral surface of the medulla oblongata and peripheral chemoreceptors in the aortic and carotid bodies [6–8]. These chemoreceptors control the respiration and heart rates to adjust the oxygen supply in the entire body. In localized hypoxia and chronic hypoxia, the tissues and cells can also sense decreases in oxygen tension through transcription factor complexes known as hypoxia-inducible factors (HIFs) to restore homeostasis [2, 66]. Hypoxia promotes angiogenesis and osteogenesis in bone by elevating the VEGF levels induced by HIF-1α in osteoblasts, as HIF-1α overexpression results in extremely dense, heavily vascularized long bones and a high level of VEGF, whereas the lack of HIF-1α in osteoblasts leads to significantly thinner and less vascularized long bones [67]. Hypoxia inhibits adipogenesis and the conditional knockout of HIF-1α in mouse embryonic fibroblasts impairs the hypoxia-mediated inhibition of adipogenesis [68]. In HIF-1α-deficient mice, hematopoietic stem cells (HSCs) in bone marrow lose cell cycle quiescence, and HSC numbers decrease [69]. B lymphocyte development is abnormal in HIF-1α-deficient chimeric mice, inducing autoimmunity [70].
Below, we introduce the emerging evidence of the involvement of hypoxia in mammary development and lactation.
Mammary development and lactation
The development of the mammary gland can be divided into three stages, embryo, puberty, and pregnancy, and the major development occurs during postnatal stages. In mice, two milk lines form between the fore and hind limbs of an E 10.5 embryo, and five pairs of placodes arise along each of the two milk lines at E 11.5. The placodes then invaginate into the underlying mesenchyme to form small bulb-shaped buds. The mammary epithelial cells of the buds proliferate from E 15.5 to form sprouts that penetrate to the underlying fat pad and form the rudimentary ductal tree and teats [71, 72]. Canonical Wnt signaling plays an important role during embryonic mammary development: Wnt 6, Wnt 10a, and Wnt 10b are expressed during this period, and the inhibition of Wnt signaling results in impaired placode formation [72, 73]. From birth to puberty, the mammary gland first undergoes isometric growth (with the same rate as the body), followed by allometric growth (2-4X faster than body fat deposition). Robust duct branching then begins at the onset of puberty. Terminal end buds (TEBs) form from the tip of the rudimentary ductal tree of the mouse mammary gland and drive pubertal mammary development. TEBs are highly proliferative, penetrating further into the fat pad, and the bifurcations of TEBs form the primary ducts. TEBs continue to invade into the fat pad until the primary ducts reach the border of the fat pad, and the secondary ducts then branch laterally from the primary ducts until the fat pad is filled with extensive ducts [74]. In ruminants, the terminal ductal lobular units (TDLUs) are the characteristic structures of postpubertal mammary development. During this stage, estrogen and growth hormone regulate duct branching, as the knock-out of estrogen receptor (ER) α or growth hormone receptor was found to impair duct development during puberty [75, 76]. During the early stage of pregnancy, short tertiary side-branches form along the ductal system developed during puberty, a process that is regulated by progesterone, progesterone receptor, and downstream Wnt 4 signaling [77, 78]. The mammary epithelial cells then proliferate rapidly to form a lobuloalveolar structure within the ductal branches. Prolactin is important for alveolar morphogenesis, as the knock-out of prolactin receptor or STAT5a, a major prolactin downstream-signaling molecule, results in lobuloalveolar defects [79, 80]. During late pregnancy, the mammary epithelial cells differentiate, and lactogenesis occurs under the synergistic effects of prolactin, glucocorticoids, and insulin [81]. Milk protein genes and lipogenic genes are expressed, and lipid droplets form in the epithelial cells. At the onset of lactation, the peak blood levels of lactogenic hormones and withdrawal of progesterone lead to copious milk secretion [81]. Extensive angiogenesis to supply nutrients is associated with all stages of mammary development [82]; in particular, extensive capillaries form a basket-like architecture to surround the alveoli during lobuloalveolar development. The vascular density doubles from day 18 of pregnancy to day 5 of lactation in mice [83]. In consistent with vascular development, the expression of VEGF and VEGF receptor in the rodent mammary gland increases dramatically during pregnancy and lactation and decreases during involution [84].
Oxygen uptake in the mammary gland during mammary development and lactation
The mammary gland has high metabolic rates during development and lactation and is thus considered to be a benign, highly regulated tumor [85]. Although extensive studies have been performed in the pathology of hypoxia in breast cancer, limited attention has been given to oxygen utilization in normal mammary development and lactation, and reports on mammary oxygen uptake have been largely limited to only a few early studies [86–88]. The average O2 uptake of a lactating mammary gland is 0.51-0.73 μmol/min/g in goats and 2.06 in rats [86–88]. Mammary O2 uptake is steadily increased during late pregnancy and reaches the highest levels in early lactation [89]. The O2 uptake in lactating goats is twice that in the preparturient goat, and there is a correlation between mammary O2 uptake and milk secretion [87, 88]. Starvation results in the virtual cessation of milk production, with a 75% reduction in mammary oxygen uptake [86], whereas growth hormone administration increases mammary oxygen uptake [90]. In 10-wk virgin mice, the pO2 level in the mammary fat pad is, on average, 13.0 mm Hg (~2%), which is considerably lower than in muscle (29.1 mm Hg, ~4%) [91]. However, a recent study using phosphonated trityl probes in mice reported an average mammary gland tissue pO2 of 52 mmHg [92], which is consistent with the pO2 value reported in normal breast tissue [93]. Nevertheless, it is likely that the mammary gland develops chronic hypoxia during the rapid mammary development in late pregnancy and in early lactation because the oxygen consumption increases in these periods to meet the increased metabolic rates.
To examine possible hypoxic conditions in the mammary gland during mammary development, we recently injected the hypoxia marker pimonidazole HCl into mice from the virgin state to the early lactation state. Pimonidazole HCl is a chemical that forms adducts with thiol groups in proteins, peptides, and amino acids in hypoxic cells (http://www.hypoxyprobe.com/), and these pimonidazole adducts can be detected with specific antibodies. Immunohistochemical staining of the mouse mammary glands from different stages showed a hypoxic mammary gland in all examined stages, but the staining was stronger during late pregnancy and early lactation (Shao and Zhao, unpublished preliminary observations), indicating a more hypoxic condition in these stages.
Emerging evidence of a role of hypoxia in mammary development and lactation
Consistent with the hypoxic condition in the mammary gland during development and lactation, there is emerging evidence that supports possible physiological roles of hypoxia in these processes.
1) Selective deletion of HIF-1α in the mouse mammary gland results in impaired mammary development and lactation
The most compelling evidence of hypoxia’s role in mammary development and lactation is from a targeted gene-knockout study by Seagroves et al. (2003) [94]. Because the whole-genome knockout of the HIF-1α gene is embryonic lethal, Seagroves et al. specifically removed the HIF-1α gene from the mammary epithelial cells (MECs) using mouse mammary tumor virus (MMTV) promoter-directed cre-lox technology. No morphological defects were observed in the HIF-1α-/- glands until day 15 of pregnancy when the mammary gland is well into secretory differentiation. By day 15 of pregnancy, striking histological differences became obvious between the wild-type and HIF-1α-/- glands. In particular, the HIF-1α-/- glands had smaller alveoli with no protein or lipid droplets due to impaired mammary differentiation. In addition, the expression of milk proteins β-casein and α-lactalbumin was decreased by over 50% in these glands. Surprisingly, the HIF-1α-/- glands showed no abnormality in microvessel pattering and vascular density, despite the important role of hypoxia in angiogenesis [94]. However, this observation could be explained by HIF-1α only being deleted in MECs and not in other cell types, such as endothelial and stroma cells, and these cells could still respond to the hypoxia conditions and retain intact angiogenesis.
Furthermore, consistent alveolar defects and trapped large lipid droplets were observed in the HIF-1α-/- glands during lactation [94]. The glands weighed ~50% of the wild-type glands at mid-lactation; additionally, the HIF-1α-/- animals produced less milk than the wild-type controls, and their milk was more viscous and contained significantly elevated fat and ion contents.
These data clearly indicate that, although the expression of HIF-1α in MECs is not required for early mammary development (mainly ductal morphogenesis), it is essential for mammary secretory differentiation, milk production, and lipid secretion, implying a role for hypoxia in these physiological processes.
2) Hypoxia increases glucose uptake and GLUT1 expression in MECs
As in all mammalian cells, glucose is an important source of energy and NADH and also serves as a substrate for lipid, protein, and nucleotide syntheses in MECs. Glucose is also the major and an essential precursor of lactose synthesis in the lactating MECs. Mammary glucose uptake increases gradually from late pregnancy and peaks at early lactation [87, 89], and mammary glucose transport activity increases approximately 40-fold from the virgin state to mid-lactation state in mice [95]. Glucose uptake in the mammary gland is mediated by facilitative glucose transporters (GLUTs) [31, 96]. Mammary cells mainly express GLUT1, GLUT8, and GLUT 12, with GLUT1 being the predominant isoform [97], and there is a dramatic increase in mammary GLUT expression from late pregnancy to early lactation [97], concomitantly with mammary glucose uptake. We originally hypothesized that the lactogenic hormones (prolactin, glucocorticoids, insulin, and estrogen) are responsible for stimulating GLUT expression during lactogenesis. However, our recent study challenged this hypothesis because these hormones failed to stimulate GLUT expression in bovine mammary explants and primary MECs, even though they were able to dramatically stimulate the expression of milk protein and lipogenic genes [98].
We, thus, hypothesized that the mechanism underlying the increase in GLUT expression in MECs during the transition period from pregnancy to lactation involves hypoxia signaling through hypoxia inducible factor-1α (HIF-1α). To test this hypothesis, we recently studied the effects of hypoxia on GLUT expression in bovine MECs. Hypoxia (below 5% O2) significantly stimulated glucose uptake and GLUT1 mRNA and protein expression in bovine MECs yet decreased GLUT8 mRNA expression in these cells (Shao and Zhao, unpublished observations). A robust induction of HIF-1α protein was observed in the bovine MECs, consistent with the observation in mouse MECs [94]. Furthermore, an siRNA against HIF-1α completely abolished the up-regulation of GLUT1 by hypoxia but had no effect on GLUT8 expression (Shao and Zhao, unpublished observations).
Consistent with our study, the expression of GLUT1 in mouse MECs is HIF-1α-dependent in a stage-dependent manner in vivo. GLUT1 expression decreases by 60% in HIF-1α-/- glands by day 16 of pregnancy, whereas no difference was observed at mid-lactation [94]. In addition, it has been shown that prolonged hypoxia stimulates GLUT1 expression in bovine endothelial cells [99, 100].
Taken together, the above evidence clearly show that MECs and mammary endothelial cells are responsive to hypoxia (as high as 5% O2) through HIF-1α.
3) Possible interactions of HIF-1α with other signaling pathways in the mammary gland
The signal transducer and activator of transcription 5 (STAT5) is essential for mammary gland differentiation and lactation [101, 102] and is mainly activated by prolactin and its receptor in the mammary gland. After binding prolactin, the prolactin receptor phosphorylates and activates Janus kinase (JAK) 2. JAK2 then phosphorylates STAT5, and STAT5 dimerizes and translocates to the nucleus to stimulate target genes’ expression [103, 104]. In addition to the prolactin pathway, STAT5 can also be activated by epidermal growth factor (EGF), growth hormone, insulin growth factor (IGF), estrogen, and progesterone signaling pathways in the mammary gland [105]. STAT5 controls the population of luminal progenitor cells that will differentiate to alveolar cells [106, 107]. During lactogenesis and lactation, the prolactin-STAT5 pathway controls the expression of milk protein genes and lipogenic genes [81, 108]. STAT5-null mice have impaired mammary alveologenesis due to a reduction in the mammary luminal progenitor cell population and exhibit impaired milk protein gene expression [109]. It has been reported that hypoxia and HIF-1α can induce STAT5 phosphorylation and enhance its DNA-binding activity in mammary epithelial cells and breast cancer cells [110, 111]. Thus, hypoxia may be involved in mammary development and lactation by regulating STAT5 activity.
Notch signaling responds to extrinsic or intrinsic developmental cues and regulates multiple cellular processes, such as stem cell maintenance, cell fate specification, and differentiation [112]. Upon ligand binding, the Notch protein is cleaved by presenilin/γ-secretase to release the active intracellular domain (ICD), which translocates to the nucleus to regulate the transcription of target genes [113]. In the mammary gland, Notch represses mammary stem cells expansion in the basal cell compartment [114] and promotes luminal cell fate specification and prevents myoepithelial cell lineage during pregnancy [114, 115]. Hypoxia prevents differentiation in various stem and precursor cells, and research has shown that HIF-1α interacts with the Notch ICD to activate Notch target genes, inhibiting the differentiation of myogenic and neutral precursor cells [116]. In addition, FIH negatively regulates Notch by hydroxylating two asparagine residues of ICD [117]. Therefore, hypoxia and Notch signaling may cross-talk to regulate cell differentiation in the mammary gland.
Wnt proteins are involved in multiple events during embryogenesis and adult tissue development, with effects on cell fate specification, differentiation, mitogenic stimulation, and stem cell self-renewal [118]. The Wnt signaling pathways include the canonical pathway involving β-catenin and noncanonical pathways; the canonical pathway has been investigated intensively and best characterized. Wnt proteins bind to the cell surface receptor Frizzled in conjunction with low-density lipoprotein receptor-related proteins (LRPs), which transduce the signal to intracellular proteins, leading to the stabilization of β-catenin. β-Catenin then translocates to the nucleus and interacts with transcription factor lymphoid enhancer-binding factor 1/T cell-specific transcription factor (LEF/TCF) to affect target gene transcription [118]. Wnt signaling is essential for mammary rudiment formation in embryos, as the lack of TCF or the overexpression of the Wnt inhibitor Dkk-1 impairs the formation of mammary placodes [73, 119, 120]. Wnt signals drive ductal branching during pubertal mammary development, because the deletion of LRP reduces duct branching and the overexpression of LRP increases duct branching in the virgin mouse mammary gland [121–123]. Wnt-4 works as a paracrine factor downstream of progesterone signaling during pregnancy to stimulate lobular alveolar development [77, 124]. During mammary development, both ductal and alveolar epithelial cells originate from mammary stem cells, and Wnt signaling serves as a rate-limiting self-renewal signal to maintain mammary stem cells [125]. Most stem cells reside in hypoxic niches, and research has shown that Wnt signaling is modulated by hypoxia in embryonic stem cells and neural stem cells by enhancing β-catenin activation and increasing LEF/TCF proteins [126]. Such interactions may also exist in the mammary gland.
Conclusion
Increased oxygen and energy requirements, nutrient stress, extensive angiogenesis, and metabolic switching to glycolysis [127] in the mammary gland during the transition period from pregnancy to lactation make hypoxia a possible regulator of these processes. The possible effects of hypoxia on the mammary gland include, but not limit to the stimulations of: i) glucose uptake through enhancing the expression of GLUT1, ii) angiogenesis through enhancing the expression of VEGF, iii) anaerobic glycolysis through up-regulating the expression of several key glycolytic genes, including HKs, LDHA, and PDK1, and iv) mammary development, differentiation and lactation through regulation of Notch, Wnt and STAT5 signaling pathways (Figure 3).
Schematic diagram of proposed effects of hypoxia on the mammary gland. Hypoxia stabilizes the hypoxia-inducible factor-1α (HIF-1α), which stimulates: i) glucose uptake through enhancing the expression of facilitative glucose transporter 1 (GLUT1), ii) angiogenesis through enhancing the expression of vascular endothelial growth factor (VEGF), iii) anaerobic glycolysis through up-regulating the expression of several key glycolytic genes, including hexokinases 1 and 2 (HKs), lactate dehydrogenase A (LDHA), and pyruvate dehydrogenase kinase 1 (PDK1), and iv) mammary development, differentiation and lactation through regulation of Notch, Wnt and STAT5 signaling pathways.
However, based on above evidence, it is important to note that, although HIF-1α is clearly required in normal mammary development and lactation, there is a possibility that hypoxia per se may not be the major or only stimulus of HIF-1α activity in the mammary gland. As mentioned above, in addition to being regulated by hypoxia, HIF-1α stability has been shown to be regulated by other stimuli, including nitric oxide, reactive oxygen species, nutrient stress, and glycolytic intermediates [10, 21–23]. Furthermore, several studies have shown that hypoxia causes mammary epithelial disorganization and induces a cancer cell-like phenotype in human MECs [128–130]. Thus, to link an effect of hypoxia to mammary development and lactation, it is essential to quantitatively measure oxygen tension changes in the mammary gland from pregnancy to lactation. Our hypothesis is that a small degree of the hypoxic condition is involved in mammary development and lactation from late pregnancy to early lactation, whereas more severe hypoxia is involved in breast cancer development.
Abbreviations
- FIH:
-
Factor inhibiting HIF-1
- GLUT:
-
Facilitative glucose transporter
- HIF:
-
Hypoxia-inducible factor
- HRE:
-
Hypoxia response element
- LDHA:
-
Lactate dehydrogenase A
- MECs:
-
Mammary epithelial cells
- PDC:
-
Pyruvate dehydrogenase complex
- PDK1:
-
Pyruvate dehydrogenase kinases 1
- PHD:
-
Prolyl hydroxylase domain protein
- SRE:
-
Serum responsive element
- STAT5:
-
Signal transducer and activator of transcription 5
- TCA:
-
Tricarboxylic acid cycle.
References
Rich PR: The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans. 2003, 31: 1095-1105. 10.1042/BST0311095.
Cassavaugh J, Lounsbury KM: Hypoxia-mediated biological control. J Cell Biochem. 2011, 112: 735-744. 10.1002/jcb.22956.
Braun RD, Lanzen JL, Snyder SA, Dewhirst MW: Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol. 2001, 280: H2533-2544.
Aragones J, Fraisl P, Baes M, Carmeliet P: Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009, 9: 11-22. 10.1016/j.cmet.2008.10.001.
Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D: HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001, 15: 2445-2453.
Prabhakar NR: O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters?. Exp Physiol. 2006, 91: 17-23. 10.1113/expphysiol.2005.031922.
Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR: Oxygen sensing in the body. Prog Biophy Mol Biol. 2006, 91: 249-286. 10.1016/j.pbiomolbio.2005.07.001.
Prabhakar NR: Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013, 591: 2245-2257.
**a X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL: Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci USA. 2009, 106: 4260-4265. 10.1073/pnas.0810067106.
Prabhakar NR, Semenza GL: Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012, 92: 967-1003. 10.1152/physrev.00030.2011.
Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995, 92: 5510-5514. 10.1073/pnas.92.12.5510.
Jiang BH, Rue E, Wang GL, Roe R, Semenza GL: Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996, 271: 17771-17778. 10.1074/jbc.271.30.17771.
Semenza GL: Hypoxia-inducible factors in physiology and medicine. Cell. 2012, 148: 399-408. 10.1016/j.cell.2012.01.021.
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001, 107: 43-54. 10.1016/S0092-8674(01)00507-4.
Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH: Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000, 275: 25733-25741. 10.1074/jbc.M002740200.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999, 399: 271-275. 10.1038/20459.
Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004, 5: 343-354. 10.1038/nrm1366.
Mahon PC, Hirota K, Semenza GL: FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15: 2675-2686. 10.1101/gad.924501.
Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK: FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002, 16: 1466-1471. 10.1101/gad.991402.
Kaelin WG, Ratcliffe PJ: Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008, 30: 393-402. 10.1016/j.molcel.2008.04.009.
Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A: Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005, 280: 41928-41939. 10.1074/jbc.M508718200.
Lu H, Forbes RA, Verma A: Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002, 277: 23111-23115. 10.1074/jbc.M202487200.
Hirota K, Semenza GL: Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun. 2005, 338: 610-616. 10.1016/j.bbrc.2005.08.193.
Loboda A, Jozkowicz A, Dulak J: HIF-1 and HIF-2 transcription factors--similar but not identical. Mol Cells. 2010, 29: 435-442. 10.1007/s10059-010-0067-2.
Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, et al: Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003, 17: 271-273.
Maynard MA, Evans AJ, Hosomi T, Hara S, Jewett MA, Ohh M: Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB J. 2005, 19: 1396-1406. 10.1096/fj.05-3788com.
Ryan HE, Lo J, Johnson RS: HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998, 17: 3005-3015. 10.1093/emboj/17.11.3005.
Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D, Carmeliet P: Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc Res. 2003, 60: 569-579. 10.1016/j.cardiores.2003.07.003.
Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT: Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006, 281: 25703-25711. 10.1074/jbc.M602329200.
Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, et al: Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003, 35: 331-340. 10.1038/ng1266.
Zhao FQ, Keating AF: Functional properties and genomics of glucose transporters. Curr Genomics. 2007, 8: 113-128. 10.2174/138920207780368187.
Zhang JZ, Behrooz A, Ismail-Beigi F: Regulation of glucose transport by hypoxia. Am J Kidney Dis. 1999, 34: 189-202. 10.1016/S0272-6386(99)70131-9.
Baumann MU, Zamudio S, Illsley NP: Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2007, 293: C477-485. 10.1152/ajpcell.00075.2007.
Ren BF, Deng LF, Wang J, Zhu YP, Wei L, Zhou Q: Hypoxia regulation of facilitated glucose transporter-1 and glucose transporter-3 in mouse chondrocytes mediated by HIF-1alpha. Joint Bone Spine. 2008, 75: 176-181. 10.1016/j.jbspin.2007.05.012.
Behrooz A, Ismail-Beigi F: Dual control of glut1 glucose transporter gene expression by hypoxia and by inhibition of oxidative phosphorylation. J Biol Chem. 1997, 272: 5555-5562. 10.1074/jbc.272.9.5555.
Ebert BL, Firth JD, Ratcliffe PJ: Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem. 1995, 270: 29083-29089. 10.1074/jbc.270.49.29083.
Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A: Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996, 271: 32529-32537. 10.1074/jbc.271.51.32529.
Semenza GL: Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med. 1996, 6: 151-157. 10.1016/1050-1738(96)00039-4.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3: 177-185. 10.1016/j.cmet.2006.02.002.
Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, Burke LM: Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab. 2006, 290: E380-388.
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL: HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007, 129: 111-122. 10.1016/j.cell.2007.01.047.
Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL: HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007, 11: 407-420. 10.1016/j.ccr.2007.04.001.
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL: Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008, 283: 10892-10903. 10.1074/jbc.M800102200.
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL: Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996, 16: 4604-4613.
Wang GL, Semenza GL: Molecular basis of hypoxia-induced erythropoietin expression. Curr Opin Hematol. 1996, 3: 156-162. 10.1097/00062752-199603020-00009.
Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE: Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991, 88: 5680-5684. 10.1073/pnas.88.13.5680.
Haase VH: Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27: 41-53. 10.1016/j.blre.2012.12.003.
Kunz M, Ibrahim SM: Molecular responses to hypoxia in tumor cells. Mol Cancer. 2003, 2: 23-10.1186/1476-4598-2-23.
Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell. 2011, 144: 646-674. 10.1016/j.cell.2011.02.013.
Erler JT, Cawthorne CJ, Williams KJ, Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C, Stratford IJ, Dive C: Hypoxia-mediated down-regulation of Bid and Bax in tumors occurs via hypoxia-inducible factor 1-dependent and -independent mechanisms and contributes to drug resistance. Mol Cell Biol. 2004, 24: 2875-2889. 10.1128/MCB.24.7.2875-2889.2004.
Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al: The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010, 120: 127-141. 10.1172/JCI40027.
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ: Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996, 379: 88-91. 10.1038/379088a0.
Airley RE, Mobasheri A: Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007, 53: 233-256. 10.1159/000104457.
Cairns RA, Harris IS, Mak TW: Regulation of cancer cell metabolism. Nat Rev Cancer. 2011, 11: 85-95. 10.1038/nrc2981.
Bristow RG, Hill RP: Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008, 8: 180-192. 10.1038/nrc2344.
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT: Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005, 1: 401-408. 10.1016/j.cmet.2005.05.001.
Yotnda P, Wu D, Swanson AM: Hypoxic tumors and their effect on immune cells and cancer therapy. Methods Mol Biol. 2010, 651: 1-29. 10.1007/978-1-60761-786-0_1.
Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM: Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003, 3: 347-361. 10.1016/S1535-6108(03)00085-0.
Wilson WR, Hay MP: Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011, 11: 393-410. 10.1038/nrc3064.
Vaupel P, Mayer A: Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26: 225-239. 10.1007/s10555-007-9055-1.
Young RJ, Moller A: Immunohistochemical detection of tumour hypoxia. Methods Mol Biol. 2010, 611: 151-159. 10.1007/978-1-60327-345-9_12.
Semenza GL: Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011, 365: 537-547. 10.1056/NEJMra1011165.
Wood IS, de Heredia FP, Wang B, Trayhurn P: Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009, 68: 370-377. 10.1017/S0029665109990206.
Wood IS, Wang B, Lorente-Cebrian S, Trayhurn P: Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human adipocytes. Biochem Biophys Res Commun. 2007, 361: 468-473. 10.1016/j.bbrc.2007.07.032.
Dunwoodie SL: The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009, 17: 755-773. 10.1016/j.devcel.2009.11.008.
Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS: Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15: 2865-2876.
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, et al: The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007, 117: 1616-1626. 10.1172/JCI31581.
Yun Z, Maecker HL, Johnson RS, Giaccia AJ: Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002, 2: 331-341. 10.1016/S1534-5807(02)00131-4.
Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T: Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010, 7: 391-402. 10.1016/j.stem.2010.06.020.
Kojima H, Gu H, Nomura S, Caldwell CC, Kobata T, Carmeliet P, Semenza GL, Sitkovsky MV: Abnormal B lymphocyte development and autoimmunity in hypoxia-inducible factor 1alpha -deficient chimeric mice. Proc Natl Acad Sci USA. 2002, 99: 2170-2174. 10.1073/pnas.052706699.
Hens JR, Wysolmerski JJ: Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005, 7: 220-224. 10.1186/bcr1306.
Robinson GW: Cooperation of signalling pathways in embryonic mammary gland development. Nat Rev Genet. 2007, 8: 963-972. 10.1038/nrg2227.
Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE: Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004, 131: 4819-4829. 10.1242/dev.01347.
Howlin J, McBryan J, Martin F: Pubertal mammary gland development: insights from mouse models. J Mammary Gland Biol Neoplasia. 2006, 11: 283-297. 10.1007/s10911-006-9024-2.
Mueller SO, Clark JA, Myers PH, Korach KS: Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002, 143: 2357-2365.
Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L: Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlap** developmental effects. Dev Biol. 2001, 229: 163-175. 10.1006/dbio.2000.9961.
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA: Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000, 14: 650-654.
Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA: A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998, 95: 5076-5081. 10.1073/pnas.95.9.5076.
Ormandy CJ, Camus A, Barra J, Damotte D, Lucas B, Buteau H, Edery M, Brousse N, Babinet C, Binart N, Kelly PA: Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997, 11: 167-178. 10.1101/gad.11.2.167.
Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L: Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11: 179-186. 10.1101/gad.11.2.179.
Neville MC, McFadden TB, Forsyth I: Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002, 7: 49-66. 10.1023/A:1015770423167.
Djonov V, Andres AC, Ziemiecki A: Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech. 2001, 52: 182-189. 10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M.
Matsumoto M, Nishinakagawa H, Kurohmaru M, Hayashi Y, Otsuka J: Pregnancy and lactation affect the microvasculature of the mammary gland in mice. J Vet Med Sci. 1992, 54: 937-943. 10.1292/jvms.54.937.
Pepper MS, Baetens D, Mandriota SJ, Di Sanza C, Oikemus S, Lane TF, Soriano JV, Montesano R, Iruela-Arispe ML: Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev Dyn. 2000, 218: 507-524. 10.1002/1097-0177(200007)218:3<507::AID-DVDY1012>3.0.CO;2-5.
Williamson DH, Evans RD, Wood SC: Tumor growth and lipid metabolism during lactation in the rat. Advances in Enzyme Regulation. Volume 27. Edited by: Weber G. 1988, Oxford and New York: Pergamon Press, 93-104.
Williamson DH, Lund P, Evans RD: Substrate selection and oxygen uptake by the lactating mammary gland. Proc Nutr Soc. 1995, 54: 165-175. 10.1079/PNS19950046.
Linzell JL: Mammary-gland blood flow and oxygen, glucose and volatile fatty acid uptake in the conscious goat. J Physiol. 1960, 153: 492-509.
Reynolds M: Mammary respiration in lactating goats. Am J Physiol. 1967, 212: 707-710.
Davis AJ, Fleet IR, Goode JA, Hamon MH, Walker FM, Peaker M: Changes in mammary function at the onset of lactation in the goat: correlation with hormonal changes. J Physiol. 1979, 288: 33-44.
Davis SR, Collier RJ, McNamara JP, Head HH, Croom WJ, Wilcox CJ: Effects of thyroxine and growth hormone treatment of dairy cows on mammary uptake of glucose, oxygen and other milk fat precursors. J Anim Sci. 1988, 66: 80-89.
Matsumoto A, Matsumoto S, Sowers AL, Koscielniak JW, Trigg NJ, Kuppusamy P, Mitchell JB, Subramanian S, Krishna MC, Matsumoto K: Absolute oxygen tension (pO(2)) in murine fatty and muscle tissue as determined by EPR. Magn Reson Med. 2005, 54: 1530-1535. 10.1002/mrm.20714.
Dhimitruka I, Bobko AA, Eubank TD, Komarov DA, Khramtsov VV: Phosphonated trityl probes for concurrent in vivo tissue oxygen and pH monitoring using electron paramagnetic resonance-based techniques. J Am Chem Soc. 2013, 135: 5904-5910. 10.1021/ja401572r.
Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, et al: Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006, 82: 699-757. 10.1080/09553000601002324.
Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W, Welm B, Wagner KU, Neville M, Johnson RS: HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development. 2003, 130: 1713-1724. 10.1242/dev.00403.
Prosser CG, Topper YJ: Changes in the rate of carrier-mediated glucose transport by mouse mammary epithelial cells during ontogeny: hormone dependence delineated in vitro. Endocrinology. 1986, 119: 91-96. 10.1210/endo-119-1-91.
Mueckler M, Thorens B: The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013, 34: 121-138. 10.1016/j.mam.2012.07.001.
Zhao FQ, Keating AF: Expression and regulation of glucose transporters in the bovine mammary gland. J Dairy Sci. 2007, 90 (Suppl 1): E76-86.
Shao Y, Wall EH, McFadden TB, Misra Y, Qian X, Blauwiekel R, Kerr D, Zhao FQ: Lactogenic hormones stimulate expression of lipogenic genes but not glucose transporters in bovine mammary gland. Domest Anim Endocrinol. 2013, 44: 57-69. 10.1016/j.domaniend.2012.09.001.
Loike JD, Cao L, Brett J, Ogawa S, Silverstein SC, Stern D: Hypoxia induces glucose transporter expression in endothelial cells. Am J Physiol. 1992, 263: C326-333.
Takagi H, King GL, Aiello LP: Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes. 1998, 47: 1480-1488. 10.2337/diabetes.47.9.1480.
Hennighausen L, Robinson GW, Wagner KU, Liu X: Develo** a mammary gland is a stat affair. J Mammary Gland Biol Neoplasia. 1997, 2: 365-372. 10.1023/A:1026347313096.
Wagner KU, Rui H: Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia. 2008, 13: 93-103. 10.1007/s10911-008-9062-z.
Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L: Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA. 1995, 92: 8831-8835. 10.1073/pnas.92.19.8831.
Gouilleux F, Wakao H, Mundt M, Groner B: Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994, 13: 4361-4369.
Furth PA, Nakles RE, Millman S, Diaz-Cruz ES, Cabrera MC: Signal transducer and activator of transcription 5 as a key signaling pathway in normal mammary gland developmental biology and breast cancer. Breast Cancer Res. 2011, 13: 220-10.1186/bcr2921.
Yamaji D, Na R, Feuermann Y, Pechhold S, Chen W, Robinson GW, Hennighausen L: Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009, 23: 2382-2387. 10.1101/gad.1840109.
Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, Britt K, Grez M, Fehse B, Desrivieres S, Groner B: Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells. 2010, 28: 928-938.
Anderson SM, Rudolph MC, McManaman JL, Neville MC: Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis!. Breast Cancer Res. 2007, 9: 204-10.1186/bcr1653.
Barash I: Stat5 in the mammary gland: controlling normal development and cancer. J Cell Physiol. 2006, 209: 305-313. 10.1002/jcp.20771.
Joung YH, Park JH, Park T, Lee CS, Kim OH, Ye SK, Yang UM, Lee KJ, Yang YM: Hypoxia activates signal transducers and activators of transcription 5 (STAT5) and increases its binding activity to the GAS element in mammary epithelial cells. Exp Mol Med. 2003, 35: 350-357. 10.1038/emm.2003.46.
Lee MY, Joung YH, Lim EJ, Park JH, Ye SK, Park T, Zhang Z, Park DK, Lee KJ, Yang YM: Phosphorylation and activation of STAT proteins by hypoxia in breast cancer cells. Breast. 2006, 15: 187-195. 10.1016/j.breast.2005.05.005.
Chiba S: Notch signaling in stem cell systems. Stem Cells. 2006, 24: 2437-2447. 10.1634/stemcells.2005-0661.
LaVoie MJ, Selkoe DJ: The notch ligands, jagged and delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments. J Biol Chem. 2003, 278: 34427-34437. 10.1074/jbc.M302659200.
Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE: Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008, 3: 429-441. 10.1016/j.stem.2008.08.001.
Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L: The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006, 293: 565-580. 10.1016/j.ydbio.2006.02.043.
Gustafsson MV, Zheng X, Pereira T, Gradin K, ** S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M: Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005, 9: 617-628. 10.1016/j.devcel.2005.09.010.
Zheng X, Linke S, Dias JM, Gradin K, Wallis TP, Hamilton BR, Gustafsson M, Ruas JL, Wilkins S, Bilton RL, et al: Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc Natl Acad Sci USA. 2008, 105: 3368-3373. 10.1073/pnas.0711591105.
Logan CY, Nusse R: The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004, 20: 781-810. 10.1146/annurev.cellbio.20.010403.113126.
Boras-Granic K, Chang H, Grosschedl R, Hamel PA: Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006, 295: 219-231. 10.1016/j.ydbio.2006.03.030.
Brennan KR, Brown AM: Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004, 9: 119-131.
Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO: The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006, 281: 35081-35087. 10.1074/jbc.M607571200.
Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO: The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PloS ONE. 2009, 4: e5813-10.1371/journal.pone.0005813.
Zhang J, Li Y, Liu Q, Lu W, Bu G: Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene. 2010, 29: 539-549. 10.1038/onc.2009.339.
Oakes SR, Hilton HN, Ormandy CJ: The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res. 2006, 8: 207-10.1186/bcr1411.
Zeng YA, Nusse R: Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010, 6: 568-577. 10.1016/j.stem.2010.03.020.
Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC: O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol. 2010, 12: 1007-1013. 10.1038/ncb2102.
Mazurek S, Weisse G, Wust G, Schafer-Schwebel A, Eigenbrodt E, Friis RR: Energy metabolism in the involuting mammary gland. In Vivo. 1999, 13: 467-477.
Whelan KA, Reginato MJ: Surviving without oxygen: hypoxia regulation of mammary morphogenesis and anoikis. Cell Cycle. 2011, 10: 2287-2294. 10.4161/cc.10.14.16532.
Whelan KA, Caldwell SA, Shahriari KS, Jackson SR, Franchetti LD, Johannes GJ, Reginato MJ: Hypoxia suppression of Bim and Bmf blocks anoikis and luminal clearing during mammary morphogenesis. Mol Biol Cell. 2010, 21: 3829-3837. 10.1091/mbc.E10-04-0353.
Vaapil M, Helczynska K, Villadsen R, Petersen OW, Johansson E, Beckman S, Larsson C, Pahlman S, Jogi A: Hypoxic conditions induce a cancer-like phenotype in human breast epithelial cells. PLoS ONE. 2012, 7: e46543-10.1371/journal.pone.0046543.
Acknowledgements
This research was supported by National Research Initiative Competitive grant 2007-35206-18037 from the USDA National Institute of Food and Agriculture (to FQZ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YS and FQZ drafted the manuscript, and FQZ revised the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shao, Y., Zhao, FQ. Emerging evidence of the physiological role of hypoxia in mammary development and lactation. J Animal Sci Biotechnol 5, 9 (2014). https://doi.org/10.1186/2049-1891-5-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2049-1891-5-9