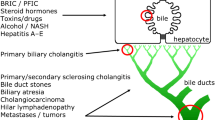
Abstract
Purpose of Review
Pruritus is a common extrahepatic symptom in various liver disorders, in particularly those with cholestatic features. This review summarizes epidemiology, pathophysiology, evidence-based therapeutic recommendations and currently investigated drugs for pruritus in hepatobiliary disorders.
Recent Findings
Recent epidemiological data suggest pruritus as a common and relevant symptom in immune-mediated liver diseases, i.e., primary biliary cholangitis (PBC) with over 70% affected patients, up to 56% suffering from chronic pruritus. The better pathophysiological understanding of hepatic pruritus has led to the identification of novel therapeutic targets, addressed in drug trials using KOR agonists, PPAR agonists, and ileal bile acid transporter inhibitors.
Summary
Hepatic itch remains among the most agonizing symptoms for affected patients and a clinical challenge for physicians. Therapeutic recommendations include a guideline-based stepwise approach starting with cholestyramine, followed by rifampicin, naltrexone, and sertraline. Bezafibrate and ileal bile acid transporter inhibitors represent promising future anti-pruritic treatment options.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368(17):1625–34. https://doi.org/10.1056/NEJMcp1208814. Well-prepared clinical case giving a good overview on classification of diseases associated with chronic pruritus.
Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. 2014;1842(7):869–92. https://doi.org/10.1016/j.bbadis.2014.02.007.
Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2014;124(1):120–33. https://doi.org/10.1097/AOG.0000000000000346.
Koulentaki M, Ioannidou D, Stefanidou M, Maraki S, Drigiannakis I, Dimoulios P, et al. Dermatological manifestations in primary biliary cirrhosis patients: a case control study. Am J Gastroenterol. 2006;101(3):541–6. https://doi.org/10.1111/j.1572-0241.2006.00423.x.
Bergasa NV, Mehlman JK, Jones EA. Pruritus and fatigue in primary biliary cirrhosis. Baillieres Best Pract Res Clin Gastroenterol. 2000;14(4):643–55. https://doi.org/10.1053/bega.2000.0109.
Hegade VS, Mells GF, Fisher H, Kendrick S, DiBello J, Gilchrist K, et al. Pruritus is common and undertreated in patients with primary biliary cholangitis in the United Kingdom. Clin Gastroenterol Hepatol. 2018;17:1379–1387.e3. https://doi.org/10.1016/j.cgh.2018.12.007.
Hönig S, Herder B, Kautz A, Trautwein C, Kremer AE. Pruritus strongly reduces quality of life in PBC patients – real life data from a large national survey. J Hepatol. 2018:S216.
McPhedran NT, Henderson RD. Pruritus and jaundice. Can Med Assoc J. 1965;92:1258–60.
Kremer AE, Oude Elferink RP, Beuers U. Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol. 2011;35(2):89–97. https://doi.org/10.1016/j.clinre.2010.10.007.
Ghent CN, Bloomer JR. Itch in liver disease: facts and speculations. Yale J Biol Med. 1979;52(1):77–82.
Beuers U, Kremer AE, Bolier R, Elferink RP. Pruritus in cholestasis: facts and fiction. Hepatology. 2014;60(1):399–407. https://doi.org/10.1002/hep.26909.
Mells GF, Pells G, Newton JL, Bathgate AJ, Burroughs AK, Heneghan MA, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology (Baltimore, Md). 2013;58(1):273–83. https://doi.org/10.1002/hep.26365.
Bergasa NV. The itch of liver disease. Semin Cutan Med Surg. 2011;30(2):93–8. https://doi.org/10.1016/j.sder.2011.04.009.
Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049–66.
Bergasa NV. The pruritus of cholestasis. J Hepatol. 2005;43(6):1078–88. https://doi.org/10.1016/j.jhep.2005.09.004.
Kremer AE, Beuers U, Oude-Elferink RP, Pusl T. Pathogenesis and treatment of pruritus in cholestasis. Drugs. 2008;68(15):2163–82.
• Verweyen E, Stander S, Kreitz K, Hoben I, Osada N, Gernart M, et al. Validation of a comprehensive set of pruritus assessment instruments: the chronic pruritus tools questionnaire PRURITOOLS. Acta Derm Venereol. 2019. https://doi.org/10.2340/00015555-3158. Important study comparing various measures to quantify chronic pruritus in humans.
Jones EA, Bergasa NV. The pruritus of cholestasis: from bile acids to opiate agonists. Hepatology. 1990;11(5):884–7.
Hegade VS, Krawczyk M, Kremer AE, Kuczka J, Gaouar F, Kuiper EM, et al. The safety and efficacy of nasobiliary drainage in the treatment of refractory cholestatic pruritus: a multicentre European study. Aliment Pharmacol Ther. 2016;43(2):294–302. https://doi.org/10.1111/apt.13449.
Beuers U, Gerken G, Pusl T. Biliary drainage transiently relieves intractable pruritus in primary biliary cirrhosis. Hepatology. 2006;44(1):280–1.
Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. https://doi.org/10.1038/nrgastro.2013.151.
•• Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–43. https://doi.org/10.1056/NEJMoa1509840. This RCT summarizes the POISE trial data and resulted in licensing of obeticholic acid as first second-line therapy in PBC.
Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67(5):1890–902. https://doi.org/10.1002/hep.29569.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65. https://doi.org/10.1016/s0140-6736(14)61933-4.
Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Richards D, Storey J, et al. BAT117213: Ileal bile acid transporter (IBAT) inhibition as a treatment for pruritus in primary biliary cirrhosis: study protocol for a randomised controlled trial. BMC Gastroenterol. 2016;16(1):71. https://doi.org/10.1186/s12876-016-0481-9.
Hegade VS, Jones DE, Hirschfield GM. Apical sodium-dependent transporter inhibitors in primary biliary cholangitis and primary sclerosing cholangitis. Dig Dis. 2017;35(3):267–74. https://doi.org/10.1159/000450988.
• Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389(10074):1114–23. https://doi.org/10.1016/S0140-6736(17)30319-7. Linerixiabt (= GSK2330672) revealed significant anti-pruritic effects in this first RCT on IBAT inhibitors.
Hegade VS, Pechlivanis A, McDonald JAK, Rees D, Corrigan M, Hirschfield GM, et al. Autotaxin, bile acid profile and effect of ileal bile acid transporter inhibition in primary biliary cholangitis patients with pruritus. Liver Int. 2019;39:967–75. https://doi.org/10.1111/liv.14069.
Al-Dury S, Wahlstrom A, Wahlin S, Langedijk J, Elferink RO, Stahlman M, et al. Pilot study with IBAT inhibitor A4250 for the treatment of cholestatic pruritus in primary biliary cholangitis. Sci Rep. 2018;8(1):6658. https://doi.org/10.1038/s41598-018-25214-0.
Mayo MJ, Pockros PJ, Jones D, Bowlus CL, Levy C, Patanwala I, et al. A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol Commun. 2019;3(3):365–81. https://doi.org/10.1002/hep4.1305.
Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, van Buuren HR, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139(3):1008–18, 18 e1. https://doi.org/10.1053/j.gastro.2010.05.009.
Murphy GM, Ross A, Billing BH. Serum bile acids in primary biliary cirrhosis. Gut. 1972;13(3):201–6.
• Kuiper EMM, van Erpecum KJ, Beuers U, Hansen BE, Thio HB, de Man RA, et al. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: results of a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52(4):1334–40. https://doi.org/10.1002/hep.23821. This RCT reveals that colesevelam is not superior to placebo in regard to pruritus treatment in cholestasis. This data challenges cholestryramine as first-line therapy.
Meixiong J, Vasavda C, Snyder SH, Dong X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc Natl Acad Sci U S A. 2019;116:10525–30. https://doi.org/10.1073/pnas.1903316116.
Meixiong J, Vasavda C, Green D, Zheng Q, Qi L, Kwatra SG, et al. Identification of a bilirubin receptor that may mediate a component of cholestatic itch. eLife. 2019;8. https://doi.org/10.7554/eLife.44116.
Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33(2):149–60.
Bergasa NV, Vergalla J, Swain MG, Jones EA. Hepatic concentrations of proenkephalin-derived opioids are increased in a rat model of cholestasis. Liver. 1996;16(5):298–302.
Swain MG, Rothman RB, Xu H, Vergalla J, Bergasa NV, Jones EA. Endogenous opioids accumulate in plasma in a rat model of acute cholestasis. Gastroenterology. 1992;103(2):630–5.
Spivey JR, Jorgensen RA, Gores GJ, Lindor KD. Methionine-enkephalin concentrations correlate with stage of disease but not pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 1994;89(11):2028–32.
Thornton JR, Losowsky MS. Opioid peptides and primary biliary cirrhosis. BMJ. 1988;297(6662):1501–4.
Bergasa NV, Sabol SL, Young WS 3rd, Kleiner DE, Jones EA. Cholestasis is associated with preproenkephalin mRNA expression in the adult rat liver. Am J Phys. 1995;268(2 Pt 1):G346–54. https://doi.org/10.1152/ajpgi.1995.268.2.G346.
Bergasa NV, Liau S, Homel P, Ghali V. Hepatic Met-enkephalin immunoreactivity is enhanced in primary biliary cirrhosis. Liver. 2002;22(2):107–13.
Boyella VD, Nicastri AD, Bergasa NV. Human hepatic met-enkephalin and delta opioid receptor-1 immunoreactivities in viral and autoimmune hepatitis. Ann Hepatol. 2008;7(3):221–6.
Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56(4):1391–400. https://doi.org/10.1002/hep.25748.
Kremer AE, Bolier R, Dixon PH, Geenes V, Chambers J, Tolenaars D, et al. Autotaxin activity has a high accuracy to diagnose intrahepatic cholestasis of pregnancy. J Hepatol. 2015;62(4):897–904. https://doi.org/10.1016/j.jhep.2014.10.041.
Robering JW, Gebhardt L, Wolf K, Kuhn H, Kremer AE, Fischer MJM. Lysophosphatidic acid activates satellite glia cells and Schwann cells. Glia. 2019;67(5):999–1012. https://doi.org/10.1002/glia.23585.
Keune WJ, Hausmann J, Bolier R, Tolenaars D, Kremer A, Heidebrecht T, et al. Steroid binding to Autotaxin links bile salts and lysophosphatidic acid signalling. Nat Commun. 2016;7:11248. https://doi.org/10.1038/ncomms11248.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–67. https://doi.org/10.1016/j.jhep.2009.04.009.
European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–72. https://doi.org/10.1016/j.jhep.2017.03.022.
Dull MM, Kremer AE. Management of chronic hepatic itch. Dermatol Clin. 2018;36(3):293–300. https://doi.org/10.1016/j.det.2018.02.008.
Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69(1):394–419. https://doi.org/10.1002/hep.30145.
• Beuers U. Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(6):318–28. https://doi.org/10.1038/ncpgasthep0521. Excellent review on the molecular mechanisms and anti-cholestatic properties of UDCA.
Grand'Maison S, Durand M, Mahone M. The effects of ursodeoxycholic acid treatment for intrahepatic cholestasis of pregnancy on maternal and fetal outcomes: a meta-analysis including non-randomized studies. J Obstet Gynaecol Can. 2014;36(7):632–41. https://doi.org/10.1016/S1701-2163(15)30544-2.
Rust C, Sauter GH, Oswald M, Buttner J, Kullak-Ublick GA, Paumgartner G, et al. Effect of cholestyramine on bile acid pattern and synthesis during administration of ursodeoxycholic acid in man. Eur J Clin Investig. 2000;30(2):135–9.
Bachs L, Pares A, Elena M, Piera C, Rodes J. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet. 1989;1(8638):574–6.
Bachs L, Pares A, Elena M, Piera C, Rodes J. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102(6):2077–80.
Tandon P, Rowe BH, Vandermeer B, Bain VG. The efficacy and safety of bile acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol. 2007;102(7):1528–36. https://doi.org/10.1111/j.1572-0241.2007.01200.x.
• Webb GJ, Rahman SR, Levy C, Hirschfield GM. Low risk of hepatotoxicity from rifampicin when used for cholestatic pruritus: a cross-disease cohort study. Aliment Pharmacol Ther. 2018;47(8):1213–9. https://doi.org/10.1111/apt.14579. Important real-life data showing that the hepatotoxicity of rifampicin is only around 5% even in long-term treatment.
Dunning KR, Anastasi MR, Zhang VJ, Russell DL, Robker RL. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS One. 2014;9(2):e87327. https://doi.org/10.1371/journal.pone.0087327.
•• Corpechot C, Chazouilleres O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378(23):2171–81. https://doi.org/10.1056/NEJMoa1714519. First RCT of bezafibrate in PBC. Aside from anti-cholestatic properties, bezafibrate may also exert anti-pruritic effects.
Reig A, Sese P, Pares A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol. 2018;113(1):49–55. https://doi.org/10.1038/ajg.2017.287.
Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. 2012;92(5):497–501. https://doi.org/10.2340/00015555-1265.
Kremer AE, Le Cleac’h A, Lemoinne S, Wolf K, De Chaisemartin L, Chollet-Martin S, et al. Antipruritic effect of bezafibrate and serum autotaxin measures in patients with primary biliary cholangitis. Gut. 2018:gutjnl-2018-317426. https://doi.org/10.1136/gutjnl-2018-317426.
Terg R, Coronel E, Sorda J, Munoz AE, Findor J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717–22.
Wolfhagen FH, Sternieri E, Hop WC, Vitale G, Bertolotti M, Van Buuren HR. Oral naltrexone treatment for cholestatic pruritus: a double-blind, placebo-controlled study. Gastroenterology. 1997;113(4):1264–9.
Carson KL, Tran TT, Cotton P, Sharara AI, Hunt CM. Pilot study of the use of naltrexone to treat the severe pruritus of cholestatic liver disease. Am J Gastroenterol. 1996;91(5):1022–3.
McRae CA, Prince MI, Hudson M, Day CP, James OF, Jones DE. Pain as a complication of use of opiate antagonists for symptom control in cholestasis. Gastroenterology. 2003;125(2):591–6.
Mayo MJ, Handem I, Saldana S, Jacobe H, Getachew Y, Rush AJ. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45(3):666–74. https://doi.org/10.1002/hep.21553.
Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98(12):2736–41. https://doi.org/10.1111/j.1572-0241.2003.08662.x.
Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J Pain Symptom Manag. 2003;26(6):1105–12.
Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25(4):1251–7. https://doi.org/10.1093/ndt/gfp588.
Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16(12):3742–7. https://doi.org/10.1681/ASN.2005020152.
Kumada H, Miyakawa H, Muramatsu T, Ando N, Oh T, Takamori K, et al. Efficacy of nalfurafine hydrochloride in patients with chronic liver disease with refractory pruritus: a randomized, double-blind trial. Hepatol Res. 2017;47(10):972–82. https://doi.org/10.1111/hepr.12830.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Miriam M. Düll declares no conflict of interest. Andreas E. Kreme has received speaker fees from CymaBay, Falk Foundation, and Intercept and has signed advisory contracts with Beiersdorf, GSK, and Intercept. He further received unrestricted research grants from Intercept.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Liver
Rights and permissions
About this article
Cite this article
Düll, M.M., Kremer, A.E. Treatment of Pruritus Secondary to Liver Disease. Curr Gastroenterol Rep 21, 48 (2019). https://doi.org/10.1007/s11894-019-0713-6
Published:
DOI: https://doi.org/10.1007/s11894-019-0713-6