Abstract
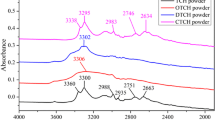
Flunixin (usually formulated as flunixin meglumine (FLN)), a common non-steroidal anti-inflammatory drug (NSAID) given to cattle, raises human health concerns when it is present in milk. Maximum residue limit (MRL) of 10 µg/kg has been set by Food Safety and Standards Authority of India (FSSAI) for FLN residues in milk. Attenuated Total Reflectance- Fourier-Transform Mid-Infrared (FT-MIR) spectroscopy in conjunction with multivariate techniques was applied to detect the presence of FLN residues in milk. Samples of FLN, pure milk as well as milk samples spiked with FLN at concentrations (1, 5, 10, 20 and 50 µg/kg) below and above the MRL were analysed. ATR-FTIR measurements were conducted in the 4000 –400 cm− 1 wavenumber range, and the wavenumber regions (2942 − 2838, 1748 − 1734 and 1149 –1012 cm− 1) were selected based on the maximum variability in the intensity of peaks and chemometrics techniques such as Principal Component Regression (PCR), Partial Least Square Regression (PLSR), Principal Component Analysis (PCA) and Soft Independent Modeling of Class Analogy (SIMCA) were applied. The study concluded that the presence of parent drug- FLN residues in milk even at 1 µg/kg level (below MRL prescribed by regulatory bodies) can be detected using ATR-FT-MIR coupled with chemometrics.
Graphical abstract





Similar content being viewed by others
Abbreviations
- FLN:
-
Flunixin meglumine
- ATR:
-
FTIR- Attenuated Total Reflectance- Fourier Transform Infrared
- NSAID:
-
Non-Steroidal Anti-Inflammatory Drug
- MIR:
-
Mid-Infrared
- FSSAI:
-
Food Safety and Standards Authority of India
- MRL:
-
Maximum Residue Limit
- LOD:
-
Limit of Detection
- LCMS:
-
Liquid Chromatography Mass Spectrometry
- LC-MS/MS:
-
Liquid Chromatography Tandem Mass Spectrometry
- HPLC:
-
High Performance Liquid Chromatography
- LFIA:
-
Lateral Flow Immunoassay
- R2 :
-
Coefficient of Determination
- PC:
-
Principal Component
- PCA:
-
Principal Component Analysis
- PCR:
-
Principal Component Regression
- PLSR:
-
Partial Least Squares Regression
- RMSE:
-
Root Mean Square Error
- SIMCA:
-
Soft Independent Modeling of Class Analogy
References
E. Daeseleire, L. Mortier, H. De Ruyck, N. Geerts, Anal. Chim. Acta. (2003). https://doi.org/10.1016/S0003-2670(03)00577-4
G.F. Sintes, R.M. Bruckmaier, O. Wellnitz, J. Dairy. Sci. (2020). https://doi.org/10.3168/jds.2020-18818
P.J. Gorden, M.D. Kleinhenz, R. Warner, P.K. Sidhu, J.F. Coetzee, J. Dairy. Sci. (2019). https://doi.org/10.3168/jds.2019-16639
O.L. Levionnois, T.K. Fosse, B. Ranheim, J. Vet. Pharmacol. Ther. (2018). https://doi.org/10.1111/jvp.12468
L. Lin, W. Jiang, L. Xu, L. Liu, S. Song, H. Kuang, Food Agric. Immunol. (2018). https://doi.org/10.1080/09540105.2017.1364710
X. Chen, S. Peng, C. Liu, X. Zou, Y. Ke, W. Jiang, Food Agric. Immunol. (2019). https://doi.org/10.1080/09540105.2019.1577365
P.K. Sidhu, R. Gehring, D.A. Mzyk, T. Marmulak, L.A. Tell, R.E. Baynes, J.E. Riviere, J. Am. Vet. Med. (2017). https://doi.org/10.2460/javma.250.2.182
L.W. Kissell, T.L. Leavens, R.E. Baynes, J.E. Riviere, G.W. Smith, J. Am. Vet. Med. 246, 118–125 (2015)
D.J. Smith, W.L. Shelver, R.E. Baynes, L. Tell, R. Gehring, M. Li, J.E. Riviere, J. Agric. Food Chem. (2015). https://doi.org/10.1021/acs.jafc.5b01509
A. Rubies, L. Guo, F. Centrich, M. Granados, Anal. Bioanal Chem. (2016). https://doi.org/10.1007/s00216-016-9679-5
FSSR, Food Safety and Standards (Contaminants, Toxins and Residues) Regulations, 2011 (Ministry of Health and Family Welfare, India, 2022). (Version 27th January 2022)
M. Pietruk, P. Jedziniak, M. Olejnik, Molecules, (2021) https://doi.org/10.3390/molecules26195892
W.F. Feely, C. Chester-Yansen, K. Thompson, J.W. Campbell, P.L. Boner, D.D. Liu, L.S. Crouch, J. Agric. Food Chem. 50, 7308–7313 (2002)
D. Douglas, K. Banaszewski, R. Juskelis, F. Al-Taher, Y. Chen, J. Cappozzo, R.S. Salter, J. Food Prot. (2012). https://doi.org/10.4315/0362-028X.JFP-11-570
R. Fan, W. Zhang, Y. **, R. Zhao, C. Yang, Q. Chen, Y. Chen, Microchim. Acta. (2020). https://doi.org/10.1007/s00604-020-04338-z
R. Saji, A. Ramani, K. Gandhi, R. Seth, R. Sharma, Food Humanity. (2024). https://doi.org/10.1016/j.foohum.2024.100239
C. Pereira, L.C. Luiz, M.J.V. Bell, V. Anjos, J. Dairy. Res. Technol. (2020). https://doi.org/10.24966/DRT-9315/100014
M. Tarapoulouzi, R. Kokkinofta, C.R. Theocharis, Food Sci. Nutr. (2020). https://doi.org/10.1002/fsn3.1603
M. Bilal, Z. **aobo, M. Arslan, H.E. Tahir, Y. Sun, R.M. Aadil, J. Near Infrared Spectrosc. (2021). https://doi.org/10.1177/0967033520979425
M. Bilal, Z. **aobo, M. Arslan, H.E. Tahir, M. Azam, Z. Junjun, S. Basheer, Vib. Spectrosc. (2020). https://doi.org/10.1016/j.vibspec.2020.103138
B. Balan, A.S. Dhaulaniya, R. Jamwal, K.K. Sodhi, S. Kelly, A. Cannavan, D.K. Singh, Vib. Spectrosc. (2020). https://doi.org/10.1016/j.vibspec.2020.103033
T.B. Coitinho, L.D. Cassoli, P.H.R. Cerqueira, H.K. da Silva, J.B. Coitinho, P.F. Machado, J. Food Sci. (2017). https://doi.org/10.1007/s13197-017-2680-y
D.G. Conceição, B.H.R. Gonçalves, F.F.D. Hora, A.S. Faleiro, L.S. Santos, S.P. Ferrão, J. Braz Chem. Soc. (2019). https://doi.org/10.21577/0103-5053.20180208
A.G. de Freitas, B.E. de Magalhães, L.A. Minho, D.J. Leão, L.S. Santos, S Augusto De Albuq. Fernandes J. Sci. Food Agric. (2021). https://doi.org/10.1002/jsfa.10799
C.G. Harshitha, N. Sharma, R. Singh, R. Sharma, K. Gandhi, B. Mann, J. Food Sci. (2023). https://doi.org/10.1007/s13197-022-05587-x
P. Jaiswal, S.N. Jha, A. Borah, A. Gautam, M.K. Grewal, G. **dal, Food Chem. (2015). https://doi.org/10.1016/j.foodchem.2014.07.010
P. Jaiswal, S.N. Jha, J. Kaur, A. Borah, H.G. Ramya, Food Chem. (2018). https://doi.org/10.1016/j.foodchem.2016.07.150
S. Jawaid, F.N. Talpur, S.T.H. Sherazi, S.M. Nizamani, A.A. Khaskheli, Food Chem. (2013). https://doi.org/10.1016/j.foodchem.2013.05.106
D.C. Ribeiro, H.A. Neto, J.S. Lima, D.C. de Assis, K.M. Keller, S.V. Campos, L.M. Fonseca, Heliyon. (2023) https://doi.org/10.1016/j.heliyon.2023.e12898
S. Sen, Z. Dundar, O. Uncu, B. Ozen, Microchem J. (2021). https://doi.org/10.1016/j.microc.2021.106207
S. Souhassou, M. Bassbasi, A. Hirri, F. Kzaiber, A. Oussama, Int. Food Res. J. 25, 1213–1218 (2018)
A.A. Spina, C. Ceniti, C. Piras, B. Tilocca, D. Britti, V.M. Morittu, J. Anim. Sci. Technol. (2022). https://doi.org/10.5187/jast.2022.e22
K. Gandhi, R. Sharma, R. Seth, B. Mann, Appl. Food Res. (2022). https://doi.org/10.1016/j.afres.2021.100035
K. Gandhi, R. Sharma, R. Seth, A. Ramani, B. Mann, Food Humanity. (2023). https://doi.org/10.1016/j.foohum.2023.10.021
V. Sonvanshi, K. Gandhi, A. Ramani, R. Sharma, R. Seth, Results Chem. (2024). https://doi.org/10.1016/j.rechem.2024.101343
R. Saji, K. Gandhi, R. Sharma, H.V. Raghu, Food Control. (2024). https://doi.org/10.1016/j.foodcont.2024.110491
L.M. Casarrubias-Torres, O.G. Meza-Márquez, G. Osorio-Revilla, T. Gallardo-Velazquez, Acta Vet. Brno. (2018). https://doi.org/10.2754/avb201887020181
D.L. Cassimiro, M. Kobelnik, C.A. Ribeiro, M.S. Crespi, N. Boralle, Thermochim Acta. (2012). https://doi.org/10.1016/j.tca.2011.11.030
M.C. Dávila-Miliani, A. Dugarte-Dugarte, R.A. Toro, J.E. Contreras, H.A. Camargo, J.A. Henao, G. Díaz, de Delgado, Cryst. Growth Des. (2020). https://pubs.acs.org/doi/https://doi.org/10.1021/acs.cgd.0c00284
Y. Xu, G. Yan, X. Wen, L. Wu, R. Deng, Q. Liang, J. He, Eur. J. Pharm. Sci. (2022). https://doi.org/10.1016/j.ejps.2021.106019
B. Balan, A.S. Dhaulaniya, R. Jamwal, A. Yadav, S. Kelly, A. Cannavan, D.K. Singh, Spectrochim Acta Mol. Biomol. Spectrosc. (2020). https://doi.org/10.1016/j.saa.2020.118628
E. Dubreil-Chéneau, Y. Pirotais, M. Bessiral, B. Roudaut, E. Verdon, J. Chromatogr. A (2011). https://doi.org/10.1016/j.chroma.2011.06.006
P. Jedziniak, T. Szprengier-Juszkiewicz, K. Pietruk, E. Śledzińska, J. Żmudzki, Anal. Bioanal Chem. (2012). https://doi.org/10.1007/s00216-012-5860-7
Acknowledgements
The first author acknowledges the financial support in the form of fellowship which was awarded by Department of Science and Technology- Innovation in Science Pursuit for Inspired Research, New Delhi (Fellow registration No. IF220211). The authors also acknowledge ICAR-National Dairy Research Institute, Karnal for providing the facilities.
Author information
Authors and Affiliations
Contributions
Rakendhu Saji: Sample preparation and analysis, data analysis, writing original draft. Kamal Gandhi: Conceptualization, Interpretation of spectral peaks, review. Rajan Sharma: Development of models, review and editing. Rajesh Bajaj: Supervision, review and editing. Bimlesh Mann: Supervision, review and editing. Akshay Ramani: Data analysis, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saji, R., Gandhi, K., Sharma, R. et al. Detection of flunixin residues in milk using ATR- FTIR spectroscopy coupled with chemometrics. Food Measure (2024). https://doi.org/10.1007/s11694-024-02686-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02686-5