Abstract
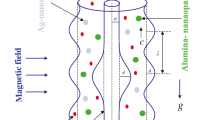
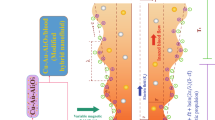
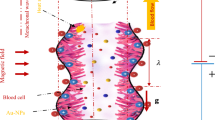
The novelty of nanoparticles in transferrals of medications and biological fluids via electrokinetic mechanism has been competently recognized. Due to the impressive role of nanoparticles suspended in blood or physiological fluids in medical fields, the current research article is planned to formulate an effective mathematical model to analyze the dynamism of bloodstream infused with hybridized nanoparticles in a non-uniform endoscopic conduit (space between two coaxial tubes) under the interactivities of electroosmosis, peristalsis, and buoyancy forces. The dual impact of heat source, Joule heating, and convectively cooling wall condition is examined. The geometrical shapes (sphere, brick, cylinder, and platelet) of nanoparticles injected into blood are accounted for in the formulation of modelled equations. The blood doped with hybridized nanoparticles is regarded as an electrolyte solution. The lubrication and Debye-Hückel linearization estimations are invoked in order to linearize the flow equations. Analytical solutions for the resulting leading equations are computed by implementing an analytical approach. The amendments in the physiognomies under variations in sundry parameters are explained through the line, bar graphs, and numerical tables. Outcomes admit that the flow of ionized blood is significantly amended across the endoscopic conduit due to the electrostatic body force. Blood is warmed or cooled with positive or negative values of Joule heating parameter. Blood is cooled with augmenting volumetric concentration of hybridized nanoparticles. The trap** phenomenon is also described by designing streamline plots. The size of confined blood boluses expands due to the thin electric double layer (EDL). The novel findings of this hemodynamic simulation furnish significant applicabilities in modelling of transportation of medications and drugs, physiological fluid mixers, testing and assessment of human diseases, detection of bacteria and viruses, etc.









Similar content being viewed by others
Abbreviations
- List of symbols:
-
Description
- a :
-
Dimensionless wave amplitude
- a ∗ :
-
Wave amplitude
- b 0 :
-
Radius of outer tube inlet
- B i :
-
Biot number
- c :
-
Wave velocity
- \(c_{i}^{\prime }s\) :
-
Expressions
- c p :
-
Specific heat capacity at constant pressure
- e :
-
Net electronic charge
- \((E_{\bar {R}}, E_{\bar {Z}})\) :
-
Electric filed components
- F :
-
Volumetric flow rate
- \(f_{i}^{\prime }s\) :
-
Expressions
- F i, F o :
-
Friction forces for inter and outer tubes
- \(_{0}\tilde {F}_{1}\) :
-
Regularized confluent hypergeometric function
- I 0, I 1 :
-
First kind modified Bessel functions of zero th and first order
- g :
-
Acceleration due to gravity
- G r :
-
Thermal Grashof number
- h ∗ :
-
Convective heat transfer coefficient
- J 0, J 1 :
-
Bessel functions of first kind of zero and first order
- k :
-
Non-uniform parameter
- \(\bar {k}\) :
-
Thermal conductivity of blood
- K B :
-
Boltzmann constant
- n :
-
Nanoparticles’ shape factor
- n 0 :
-
Average number of cations and anions
- n +, n − :
-
Number of densities of cations and anions
- p :
-
Dimensionless blood pressure
- \(\bar {P}\) :
-
Blood pressure
- P r :
-
Prandtl number
- Q :
-
Mean volume flow rate
- Q 0 :
-
Heat source
- R 0 :
-
Inner tube radius
- (r 1, r 2):
-
Dimensionless radii of inner and outer tubes
- (R 1, R 2):
-
Radii of inner and outer tubes
- R e :
-
Reynolds number
- S :
-
Joule heating parameter
- t :
-
Dimensionless time
- \(\bar {t}\) :
-
Time
- T :
-
Blood temperature
- T a :
-
Average temperature of electrolytic solution
- T 0, T 1 :
-
Constant temperatures at inner tube and outer tube
- (u,w):
-
Dimensionless velocity components in (r, z)
- \((\bar {u}, \bar {w})\) :
-
Velocity components in moving frame (\(\bar {r}\), \(\bar {z}\))
- \((\bar {U}, \bar {W})\) :
-
Velocity components in fixed frame (\(\bar {R}\), \(\bar {Z}\))
- U h s :
-
Helmholtz-Smoluchowski velocity parameter
- Y 0, Y 1 :
-
Second kind Bessel functions of zero the and first order
- \(\tilde {z}\) :
-
Valence of ions
- \(\bar {Z}\) :
-
Axial distance from inlet,
- Z ∗ :
-
Heat transfer coefficient at outer wall
- β :
-
Thermal expansion coefficient
- δ :
-
Dimensionless wave number
- 𝜖 :
-
Amplitude ratio
- ε :
-
A constant
- ε 0 :
-
Dielectric permittivity of medium
- 𝜃 :
-
Dimensionless temperature
- κ :
-
Electro-osmotic parameter
- λ :
-
Wavelength
- μ :
-
Dynamic viscosity of blood
- ρ :
-
Fluid density
- ρ e :
-
Net ionic charge density of electrolyte
- σ :
-
Electric conductivity of blood
- \(\omega _{i}^{\prime }s\) :
-
Expressions
- Φ:
-
Non-dimensional electric potential
- \(\bar {\Phi }\) :
-
Electric potential
- (ϕ 1,ϕ 2):
-
Solid volume fractions of Ag and Al2O3-NPs
- χ :
-
Heat source parameter
- ψ :
-
Stream function
- s 1 :
-
Silver nanoparticles (Ag-NPs)
- s 2 :
-
Aluminum oxide nanoparticles (Al2O3-NPs)
- f :
-
Base fluid (blood)
- nf :
-
Nano-blood
- hnf :
-
Hybrid nano-blood
References
Ijaz S, Nadeem S (2017) A biomedical solicitation examination of nanoparticles as drug agent to minimize the hemodynamics of stenotic channel. Eur Phys J Plus 132:448
Mekheimer KS, Mohamed SM, Elnaqeeb T (2016) Metallic nanaoparticles infuleunce on blood flow through a stenotic aretery. Int J Pure Appl Math 107:201–223
Gentile F, Ferrari M, Decuzzi P (2007) The transport of nanoparticles in blood vessels the effect of vessel permeability and blood rheology. Annals Biomed Eng 36:254–261
Sadaf H, Malik R (2018) Nanofluid flow analysis in the presence of slip effects and wall propetics by means of contraction and expansion. Commun Theor Phys 70:337–343
Ijaz S, Shahzadi I, Nadeem S, Saleem A (2017) A clot model examination: with impulsion of nanoparticle under infuence of variable viscosity and slip effects. Commun Theor Phys 68:667–677
Elaqeeb T, Shah NA, Mekheimer KS (2019) Hemodynamic characteristics of gold nanoparticle blood flow through a tapered stenosed vessel with variable nanofluid viscosity. BioNanoSci 9:245–255
Elmaboud YA, Mekheimer KS, Emam TG (2019) Numerical examination of gold nanoparticles as a drug carrier on peristaltic blood flow through physiological vessels: cancer therapy treatment. BioNanoSci 9:952–965
Souayeh B, Kumar KG, Reddy MG, Rani S, Hdhiri N, Alfannakh H, Rahimi-Gorjie M (2019) Slip flow and radiative heat transfer behavior of titanium alloy and ferromagnetic nanoparticles along with suspension of dusty fluid. J Mol Liq 290(15):111223
Souayeh B, Hammami F, Hdhiri N, Alam MW, Yasin E, Abuzir A (2021) Simulation of natural convective heat transfer and entropy generation of nanoparticles around two spheres in horizontal arrangement. Alex Eng J 60(2):2583–2605
Das S, Banu AS, Jana RN, Makinde OD (2022) Hall current’s impact on ionized ethylene glycol containing metal nanoparticles flowing through vertical permeable channel. J Nanofluids 11(3):444–458
Ijaz S, Nadeem S (2017) Biomedical theoretical investigation of blood mediated nanoparticles (Ag-Al2O3/blood) impact on hemodynamics of overlapped stenotic artery. J Mol Liq 248(2017):809–821
Ijaz S, Iqbal Z, Maraj EN, Nadeem S (2018) Investigate of Cu-CuO/blood mediated transportation in stenosed artery with unique features for theoretical outcomes of hemodynamics. J Mol Liq 254:421–432
Ijaz S, Nadeem S (2018) Transportation of nanoparticles investigation as a drug agent to attenuate the atherosclerotic lesion under the wall properties impact. Chaos Soliton Fract 112:52–65
Sadaf H, Iftikhar N, Akbar NS (2019) Physiological fluid flow analysis by means of contraction and expansion with addition of hybrid nanoparticles. Eur Phys J Plus 134:232
Sadaf H, Abdelsalam SI (2020) Adverse effects of a hybrid nanofluid in a wavy nonuniform annulus with convective boundary conditions. RSC Adv 10:15035
Das S, Pal TK, Jana RN (2021) Outlining impact of hybrid composition of nanoparticles suspended in blood flowing in an inclined stenosed artery undermagnetic field orientation. BioNanoSci. 11:99–115
Akram J, Akbar NS, Tripathi D (2021) A theoretical investigation on the heat transfer ability of water-based hybrid (Ag–Au) nanofluids and Ag nanofluids flow driven by electroosmotic pum** through a microchannel. Arabian J Sci Eng 46(3):2911–2927
Sharma BK, Gandhi R, Bhatti MM (2022) Entropy analysis of thermally radiating MHD slip flow of hybrid nanoparticles (Au-Al2O3/Blood) through a tapered multi-stenosed artery. Chem Phys Lett 790:139348
Ijaz S, Sadaf H, Iqbal Z (2019) Remarkable role of nanoscale particles and viscosity variation in blood flow through overlapped atherosclerotic a channel: a useful application in drug delivery. Arabian J Sci Eng 44:6241–6252
Akrama J, Akbar NS, Tripathi D (2020) Blood-based graphene oxide nanofluid flow through capillary in the presence of electromagnetic fields: a Sutterby fluid model. Microvasc Res 132:104062
Zaman A, Ali N, Khan AA (2020) Computational biomedical simulations of hybrid nanoparticles on unsteady blood hemodynamics in a stenotic artery. Math Comput Simul 169:117–132
Iftikhar N, Rehman A, Sadaf H (2021) Theoretical investigation for convective heat transfer on Cu/water nanofluid and (SiO2-copper)/water hybrid nanofluid with MHD and nanoparticle shape effects comprising relaxation and contraction phenomenon. Int Commun Heat Mass Transf 120:105012
Bhatti MM, Abdelsalam SI (2021) Bio-inspired peristaltic propulsion of hybrid nanofluid flow with tantalum (Ta) and gold (Au) nanoparticles under magnetic effects, Waves in Random and Complex Media. https://doi.org/10.1080/17455030.2021.1998728
Chakraborty S (2019) Electrokinetics with blood. Electrophoresis 40:180–189
Abdelsalam SI, Mekheimer KS, Zaher AZ (2020) Alterations in blood stream by electroosmotic forces of hybrid nanofluid through diseased artery: aneurysmal/stenosed segment. Chin J Phys 67:314–329
Mekheimer KS, Zaher AZ, Hasona WM (2020) Entropy of AC electro-kinetics for blood mediated gold or copper nanoparticles as a drug agent for thermotherapy of oncology. Chin J Phys 65:123–138
Nadeem S, Kiani MN, Saleem A, Issakhov A (2020) Microvascular blood flow with heat transfer in a wavy channel having electroosmotic effects. Electrophoresis 41(13-14):1198–1205
Das S, Pal TK, Jana RN, Giri B (2021) Significance of Hall currents on hybrid nano-blood flow through an inclined artery having mild stenosis: homotopy perturbation approach. Microvasc Res 137:104192
Das S, Pal TK, Jana RN (2021) Outlining impact of hybrid composition of nanoparticles suspended in blood flowing in an inclined stenosed artery under magnetic field orientation. BioNanoSci 11:99–115
Ellahi R, Rahman SU, Nadeem S, Vafai K (2015) The blood flow of Prandtl fluid through a tapered stenosed arteries in permeable walls with magnetic field. Commun Theor Phys 63(3):353–358
Tripathi D, Borode A, Jhorar R, Anwar Bég AO, Tiwari AK (2017) Computer modelling of electro-osmotically augmented three-layered microvascular peristaltic blood flow. Microvasc. Res 114:65–83
Tripathi D, Jhorar R, Borode A, Anwar Bég O (2018) Three-layered electro-osmosis modulated blood flow through a microchannel. Europ J Mechan B/Fluids 72:391–402
Tripathi D, Yadav A, Anwar Bég O, Kumar R (2018) Study of microvascular non-Newtonian blood flow modulated by electroosmosis. Microvasc Res 117:28–36
Reddy KV, Reddy MG, Makinde OD (2019) Thermophoresis and Brownian motion effects on magnetohydrodynamics electro-osmotic Jeffrey nanofluid peristaltic flow in asymmetric rotating microchannel. J Nanofluids 8(2):349–358
Bhatti MM, Zeeshan A, Ellahi R, Anwar Bég O, Kadir A (2019) Effects of coagulation on the two-phase peristaltic pum** of magnetized Prandtl biofluid through an endoscopic annular geometry containing a porous medium. Chin J Phys 58:222–234
Narla VK, Tripathi D, Anwar Bég O (2020) Analysis of entropy generation in biomimetic electroosmotic nanofluid pum** through a curved channel with Joule dissipation. Therm Sci Eng Prog 15:100424
Latham TW (1966) Fluid motions in a peristaltic pump PhD dissertation. Massachusetts Institute of Technology, MA
Goswami P, Chakraborty J, Bandopadhyay A, Chakraborty S (2016) Electrokinetically modulated peristaltic transport of power-law fluids. Microvasc Res 103:41–54
Tripathi D, Jhorar R, Anwar Bég O, Kadir A (2017) Electro-magneto-hydrodynamic peristaltic pum** of couple stress biofluids through a complex wavy micro-channel. J Mol Liq 236:358–367
Ranjit NK, Shit GC, Tripathi D (2018) Joule heating and zeta potential effects on peristaltic blood flow through porous micro vessels altered by electrohydrodynamic. Microvasc Res 117:74–89
Manjunatha G, Rajashekhar C, Vaidya H, Prasad KV, Makinde OD (2019) Effects wall properties on peristaltic transport of Rabinowitsch fluid through an inclined non-uniform slippery tube. Defect Diffus Forum 392:138–157
Tripathi D, Bhushan S, Anwar Bég O (2017) Analytical study of electro-osmosis modulated capillary peristaltic hemodynamics. J Mechan Med Biology 17(5):1750052
Ranjit NK, Shit GC, Tripathi D (2018) Joule heating and zeta potential effects on peristaltic blood flow through porous micro vessels altered by electrohydrodynamic. Microvasc Res 117:74–89
Noreen S, Quratulain TD (2019) Heat transfer analysis on electroosmotic flow via peristaltic pum** in non-Darcy porous medium. Thermal Science and Engineering Progress 11:254–262
Vaidya H, Rajashekhar C, Manjunatha G, Prasad KV, Makinde OD, Sreenadh S (2019) Peristaltic motion of non-Newtonian fluid with variable liquid properties in a convectively heated non-uniform tube: Rabinowitsch fluid model. J Enhanced Heat Transfer 26(3):277–294
Divya BB, Manjunatha G, Rajashekhar C, Vaidya H, Prasad KV (2021) Analysis of temperature dependent properties of a peristaltic MHD flow in a non-uniform channel: a Casson fluid model. Ain Shams Eng J 12:2181–2191
Riaz A, Awan AU, Hussain S, Khan SU, Abro KA (2021) Effects of solid particles on fluid-particulate phase flow of non-Newtonian fluid through eccentric annuli having thin peristaltic walls J Therm Anal Calorim (2021). https://doi.org/10.1007/s10973-020-10447-x
Ali A, Barman A, Das S (2022) Electromagnetic phenomena in cilia actuated peristaltic transport of hybrid nano-blood with Jeffrey model through an artery sustaining regnant magnetic field. Waves in Random and Complex Media. https://doi.org/10.1080/17455030.2022.2072533
Bhatti MM, Zeeshan A, Ellahi R (2016) Endoscope analysis on peristaltic blood flow of Sisko fluid with titanium magnetonanoparticles. Commun Biol Mdecine 78:29–41
Bhatti MM, Zeeshan A, Ijaz N (2016) Slip effects and endoscopy analysis on blood flow of particle-fluid suspension induced by peristaltic wave. J Mol Liq 218:240–245
Abdelsalam SI, Bhatti MM (2018) The impact of im**ing TiO2 nanoparticles in Prandtl nanofluid along with endoscopic and variable magnetic field effects on peristaltic blood flow. Multidiscip Model Mater Struct 14(3):530–548
Das S, Pal TK, Jana RN, Giri B (2021) Ascendancy of electromagnetic force and Hall currents on blood flow carrying Cu-Au NPs in a non-uniform endoscopic annulus having wall slip. Microvasc Res 138:104191
Das S, Barman B, Jana RN, Makinde OD (2021) Hall and ion slip currents’ impact on electromagnetic blood flow conveying hybrid nanoparticles through an endoscope with peristaltic waves. BioNanoSci. https://doi.org/10.1007/s12668-021-00873-y
Abdelsalam SI, Bhatti MM (2019) New insight into AuNp applications in tumour treatment and cosmetics through wavy annuli at the nanoscale. Sci Rep 9:260
Bhatti MM, Zeeshan A, Ellahi R, Anwar Bég O, Kadir A (2019) Effects of coagulation on the two-phase peristaltic pum** of magnetized Prandtl biofluid through an endoscopic annular geometry containing a porous medium. Chin J Phys 58:222–234
Akram J, Akbar NS (2020) Biological analysis of Carreau nanofluid in an endoscope with variable viscosity. Phys Scr 95:055201
Das S, Pal TK, Jana RN (2021) Electromagnetic hybrid nano-blood pum** via peristalsis through an endoscope having blood clotting in presence of Hall and ion slip currents. BioNanoSci. https://doi.org/10.1007/s12668-021-00853-2
Reuss FF (1809) Charge-induced flow. Proc Imp Soc Nat Moscow. 3:327–44
Wiedemann G (1852) First quantitative study of electrical endosmose. Poggendorfs Annalen 87:321–3
Shit GC, Ranjit NK, Sinha A, Anwar Bég O (2016) Electro-magnetohydrodynamic flow of biofluid induced by peristaltic wave: a non-Newtonian model. J Bionic Eng 13:436–448
Tripathi D, Sharma A, Anwar Bég O (2018) Joule heating and buoyancy effects in electroosmotic peristaltic transport of aqueous nanofluids through a microchannel with complex wave propagation. Adv Powder Technol 29:639–653
Chaube MK, Yadav A, Tripathi D, Anwar Bég O (2018) Electroosmotic flow of biorheologicalmicropolar fluids through microfluidic channels. Korea-Australia Rheology J 30(2):89–98
Jayavel P, Jhorar R, Tripathi D, Azese MN (2019) Electroosmotic flow of pseudoplastic nanoliquids via peristaltic pum**. J Braz Soc Mech Sci Eng 41:61
Noreen S, Quratulain TD (2019) Heat transfer analysis on electroosmotic flow via peristaltic pum** in non-Darcy porous medium. Therm Sci Eng Prog 11:254–262
Akram J, Akbar NS, Maraj EN (2020) A comparative study on the role of nanoparticle dispersion in electroosmosis regulated peristaltic flow of water. Alex Eng J 59:943–956
Tripathi D, Prakash J, Reddy MG, Misra JC (2021) Numerical simulation of double diffusive convection and electroosmosis during peristaltic transport of a micropolar nanofluid on an asymmetric microchannel. J Therm Anal Calorim 143(3):2499–2514
Saleem N, Munawar S, Tripathi D (2021) Thermal analysis of double diffusive electrokinetic thermally radiated TiO2-Ag/blood stream triggered by synthetic cilia under buoyancy forces and activation energy. Phys Scr 96:095218
Saleem S, Akhtar S, Nadeem S, Saleem A, Ghalambaz M, Issakhov A (2021) Mathematical study of electroosmotically driven peristaltic flow of Casson fluid inside a tube having systematically contracting and relaxing sinusoidal heated walls. Chin J Phys 71:300–311
Noreen S, Waheed S, Lu DC, Tripathi D (2021) Heat stream in electroosmotic bio-fluid flow in straight microchannel via peristalsis. Int Commun Heat Mass Transfer 123:105180
Ranjit NK, Shit GC, Tripathi D (2021) Electrothermal analysis in two-layered couple stress fluid flow in an asymmetric microchannel via peristaltic pum**. J Therm Anal Calorim 144:1325–1342
Bhatti MM, Zeeshan A, Bashir F, Sait SM, Ellahi R (2021) Sinusoidal motion of small particles through a Darcy-Brinkman-Forchheimer microchannel filled with non-Newtonian fluid under electro-osmotic forces. J Taibah Univ Sci 15(1):514–529
Nuwairan MA, Souayeh B (2022) Simulation of gold nanoparticle transport during MHD electroosmotic flow in a peristaltic micro-channel for biomedical treatment. Micromachines 13(3):374. https://doi.org/10.3390/mi13030374
Tripathi D, Yadav A, Anwar Bég O (2017) Electro-kinetically driven peristaltic transport of viscoelastic physiological fluids through a finite length capillary: mathematical modeling. Math Biosci 283:155–168
Tripathi D, Yadav A, Anwar Bég O (2017) Electro-osmotic flow of couple stress fluids in a micro-channel propagated by peristalsis. Eur Phys J Plus 132:173
Tripathi D, Sharma A, Anwar Bég O (2017) Electrothermal transport of nanofluids via peristaltic pum** in a finite micro-channel: effects of Joule heating and Helmholtz-Smoluchowski velocity. Int J Heat Mass Trans 111:138–149
Ranjit NK, Shit GC, Tripathi D (2019) Entropy generation and Joule heating of two layered electroosmotic flow in the peristaltically induced micro-channel. Int J Mech Sci 153-154:430–444
Ramesh K, Prakash J (2019) Thermal analysis for heat transfer enhancement in electroosmosis modulated peristaltic transport of Sutterby nanofluids in a microfluidic vessel. J Therm Anal Calorim 138:1311–1326
Prakash J, Siva EP, Tripathi D, Anwar Bég O (2019) Thermal slip and radiative heat transfer effects on electro-osmotic magnetonanoliquid peristaltic propulsion through a microchannel. Heat Transfer-Asian Res 48(7):2882–2908
Narla VK, Tripathi D, Anwar Bég O (2019) Electro-osmosis modulated viscoelastic embryo transport in uterine hydrodynamics: Mathematical Modelling. J Biomech Eng 141(2):021003
Narla VK, Tripathi D (2019) Electroosmosis modulated transient blood flow in curved microvessels: study of a mathematical model. Microvasc Res 123:25–34
Tanveer A, Mahmood S, Hayat T, Alsaedi A (2021) On electroosmosis in peristaltic activity of MHD non-Newtonian fluid. Alex Eng J 60:3369–3377
Ramesh K, Reddy MG, Souayeh B (2021) Electro-magneto-hydrodynamic flow of couple stress nanofluids in micro-peristaltic channel with slip and convective conditions. Appl Math Mech-Engl Ed 42:593–606. https://doi.org/10.1007/s10483-021-2727-8
Ponalagusamy R, Selvi RT, Padma R (2022) Modeling of pulsatile EMHD flow of non-Newtonian blood with magnetic particles in a tapered stenosed tube: a comparative study of actual and approximated drag force. The European Physical Journal Plus 137:230
Acknowledgements
The authors are very grateful to the honourable editor and referees for their constructive comments and valued suggestions to enhance the superiority of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical compliance
Our article has followed all protocol, rules, and ethical standards as needed for humans or animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Karmakar, P. & Ali, A. Electrothermal blood streaming conveying hybridized nanoparticles in a non-uniform endoscopic conduit. Med Biol Eng Comput 60, 3125–3151 (2022). https://doi.org/10.1007/s11517-022-02650-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02650-9