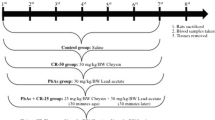
Abstract
Lead is one of the cursed substances that threaten all human life. Lead poisoning can occur through food or water contaminations and it is hard to be detected. This incognito metal accumulates over time and resides in the liver, kidneys, and brain tissues leading to serious medical conditions, affecting organ functions, causing failure, kidney tubule degeneration, and destroying neuronal development. However, known metal chelators have bad negative effects. Asparagus officinalis (AO) is a promising herb; its root extract exhibited antioxidant, antiapoptotic, protective, and immunomodulatory activities. Inspired by those reasons, this study investigated to which extent Asparagus extract affected male mice’s renal toxicity caused by lead acetate (LA) and antioxidant defense system. This work screened for its nephroprotective activity in four mouse groups: negative and positive control, LA group with renal injury, and diseased but pretreated mice with AO extract (AOE). Kidney index and kidney function biomarkers were evaluated. Antioxidant activities, lipid peroxidation, superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), nitric oxide (NO), and reduced glutathione (GSH) were also tested. Furthermore, inflammatory cytokine (tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β), and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)), inducible nitric oxide synthase (iNOS), renal pro-apoptotic protein (Bax), antiapoptotic protein (Bcl-2), and caspase-3 levels were evaluated. The results showed that LA administration induced oxidative stress, renal inflammation, apoptosis, and renal histopathological alteration. However, due to its antioxidant activities, AOE was found to restrain oxidative stress, therefore preventing inflammation and apoptosis. Collectively, AOE perfectly clogged lead poisoning sneaking, stopped the bad deterioration, and succeeded to protect kidney tissues from toxicity, inflammation, and apoptosis.






Similar content being viewed by others
Data availability
All relevant data are within the paper.
References
Abdel Moneim AE (2013) The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol Disord Drug Targets. 12(6):830–841. https://doi.org/10.2174/18715273113129990081
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS One 11(7):e0158965
Abdel Moneim AE, Dkhil MA, Al-Quraishy S (2011) The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater 194:250–5 (S0304-3894(11)00978-2)
Abdel-Wahhab MA, Abdel-Azim SH, El-Nekeety AA (2008) Inula crithmoides extract protects against ochratoxin A-induced oxidative stress, clastogenic and mutagenic alterations in male rats. Toxicon 52(4):566–73 (S0041-0101(08)00428-5)
Abo-El-Sooud K, Abd-Elhakim YM, Hashem MMM, El-Metwally AE, Hassan BA, El-Nour HHM (2023) Ameliorative effects of quercetin against hepatic toxicity of oral sub-chronic co-exposure to aluminum oxide nanoparticles and lead-acetate in male rats. Naunyn Schmiedebergs Arch Pharmacol 396(4):737–747. https://doi.org/10.1007/s00210-022-02351-y
Adouni K, Zouaoui O, Chahdoura H, Thouri A, Lamine JB, Santos-Buelga C et al (2018) In vitro antioxidant activity, α-glucosidase inhibitory potential and in vivo protective effect of Asparagus stipularis Forssk aqueous extract against high-fructose diet-induced metabolic syndrome in rats. J Funct Foods 47:521–530. https://doi.org/10.1016/j.jff.2018.06.006
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Agarwal S, Roy S, Ray A, Mazumder S, Bhattacharya S (2009) Arsenic trioxide and lead acetate induce apoptosis in adult rat hepatic stem cells. Cell Biol Toxicol 25(4):403–413. https://doi.org/10.1007/s10565-008-9094-6
Albarakati AJA, Baty RS, Aljoudi AM, Habotta OA, Elmahallawy EK, Kassab RB et al (2020) Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol Biol Rep 47(4):2591–2603. https://doi.org/10.1007/s11033-020-05346-1
Alhusaini AM, Fadda LM, Hasan IH, Ali HM, Badr A, Elorabi N et al (2020) Role of some natural anti-oxidants in the down regulation of Kim, VCAM1, Cystatin C protein expression in lead acetate-induced acute kidney injury. Pharmacol Rep 72(2):360–367. https://doi.org/10.1007/s43440-020-00072-8
Almeer RS, AlBasher GI, Alarifi S, Alkahtani S, Ali D, Abdel Moneim AE (2019) Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci Rep 9(1):5825. https://doi.org/10.1038/s41598-019-42368-7
Al-Megrin WA, Soliman D, Kassab RB, Metwally DM, Ahmed EAM, El-Khadragy MF (2020) Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front Physiol 11:64. https://doi.org/10.3389/fphys.2020.00064
Alyami NM, Almeer R, Alyami HM (2023) Protective effects of Asparagus officinalis (asparagus) against lead toxicity in mice. Environ Sci Pollut Res Int 30(7):18718–18730. https://doi.org/10.1007/s11356-022-23540-5
Ansar S, AlGhosoon HT, Hamed S (2015) Evaluation of protective effect of rutin on lead acetate-induced testicular toxicity in Wistar rats. Toxin Rev 34(4):195–199. https://doi.org/10.3109/15569543.2015.1136333
Aqeel T, Gurumallu SC, Bhaskar A, Hashimi SM, Lohith NC, Javaraiah R (2021) Protective role of flaxseed lignan secoisolariciresinol diglucoside against lead-acetate-induced oxidative-stress-mediated nephrotoxicity in rats. Phytomedicine plus 1(3):100038. https://doi.org/10.1016/j.phyplu.2021.100038
Azadbakht M, Asghari M, NowroozpoorDailami K, Davoodi A, Ahmadi A (2020) The preventive effects of Asparagus officinalis extract on sodium selenite-induced cataractogenesis in experimental animal models. Evid Based Complement Alternat Med 2020:3708730. https://doi.org/10.1155/2020/3708730
Balaji K, Vijayakumar J, Sankaran PK, Senthilkumar S, Vijayaraghavan R, Selvaraj J et al (2021) Molecular studies on the nephroprotective potential of Celastrus paniculatus against lead-acetate-induced nephrotoxicity in experimental rats: role of the PI3K/AKT signaling pathway. Molecules 26(21):6647
Baty RS, Hassan KE, Alsharif KF, El-Hennamy RE, Elmahallawy EK, Hafez MM et al (2020) Neuroprotective role of luteolin against lead acetate-induced cortical damage in rats. Hum Exp Toxicol 39(9):1200–1212. https://doi.org/10.1177/0960327120913094
Bhatnagar M, Sisodia SS, Bhatnagar R (2005) Antiulcer and antioxidant activity of Asparagus racemosus Willd and Withania somnifera Dunal in rats. Ann N Y Acad Sci 1056:261–78 (1056/1/261)
Bhatnagar M, Meena P, Satyendra B, Chetan J (2013) Neuroprotective response of the hippocampus region of the brain to Withania somnifera and Asparagus racemosus root extract: an in vitro study. J Med Plants Res 7:2259–2264. https://doi.org/10.5897/jmpr12.497
Bhattacharjee A, Kulkarni VH, Chakraborty M, Habbu PV, Ray A (2021) Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon 7(1):e05921 (S2405-8440(21)00026-8)
Bienias B, Zajaczkowska M, Borzecka H, Sikora P, Wieczorkiewicz-Plaza A, Wilczynska B (2015) Early markers of tubulointerstitial fibrosis in children with idiopathic nephrotic syndrome: preliminary report. Medicine (Baltimore) 94(42):e1746 (00005792-201510030-00039)
Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B (2007) Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 293(2):616–23. https://doi.org/10.1152/ajprenal.00507.2006
Dey P (2018) Basic and advanced laboratory techniques in histopathology and cytology. Springer Nature, Singapore
Dkhil MA, Al-Khalifa MS, Al-Quraishy S, Zrieq R, Abdel Moneim AE (2016) Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model. Drug Des Devel Ther 10:1847–1856. https://doi.org/10.2147/DDDT.S105511
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS (1998) Disruption of redox homeostasis in the transforming growth factor-α/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem 273(25):15846–15853
Fadda A, Barberis A, Sanna D (2018) Influence of pH, buffers and role of quinolinic acid, a novel iron chelating agent, in the determination of hydroxyl radical scavenging activity of plant extracts by electron paramagnetic resonance (EPR). Food Chem 240:174–182. https://doi.org/10.1016/j.foodchem.2017.07.076
Gargouri M, Soussi A, Akrouti A, Magne C, El Feki A (2018) Ameliorative effects of Spirulina platensis against lead-induced nephrotoxicity in newborn rats: modulation of oxidative stress and histopathological changes. EXCLI J 17(215–232):2017–1016
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids (Research Support, U.S. Gov’t, P.H.S.). Anal Biochem 126(1):131–8
Hasanein P, Riahi H (2018) Preventive use of berberine in inhibition of lead-induced renal injury in rats. Environ Sci Pollut Res Int 25(5):4896–4903. https://doi.org/10.1007/s11356-017-0702-y[pii]
Hossain M, Sharmin F, Akhter S, Bhuiyan M, Shahriar M (2012) Investigation of cytotoxicity and In-vitro antioxidant activity of Asparagus racemosus root extract. Int Curr Pharmaceut J 1:250–257. https://doi.org/10.3329/icpj.v1i9.11615
Hou G, Surhio MM, Ye H, Gao X, Ye Z, Li J et al (2019) Protective effects of a Lachnum polysaccharide against liver and kidney injury induced by lead exposure in mice. Int J Biol Macromol 124:716–723 (S0141-8130(18)34161-8)
Ilesanmi OB, Agoro E-YS (2022) Ameliorative effect of Trevo dietary supplement against lead acetate nephrotoxicity. Iran J Toxicol 16(1):35–42. https://doi.org/10.32598/ijt.16.1.857.1
Ito T, Goto K, Takanari J, Miura T, Wakame K, Nishioka H et al (2014) Effects of enzyme-treated asparagus extract on heat shock protein 70, stress indices, and sleep in healthy adult men. J Nutr Sci Vitaminol (Tokyo) 60(4):283–90. https://doi.org/10.3177/jnsv.60.283. (DN/JST.JSTAGE/jnsv/60.283 [pii])
Kedir MW, Dubiwak DA, Ahmed TE (2022) Nephroprotective Effect of Asparagus africanus Lam. Root Extract against Gentamicin-Induced Nephrotoxicity in Swiss Albino Mice. J Toxicol 2022:1–8. https://doi.org/10.1155/2022/8440019
Kiernan J (2015) Histological and histochemical methods. Theory and practice, 5th edn. Scion Publishing Ltd, p 571
Kongkaneramit L, Witoonsaridsilp W, Peungvicha P, Ingkaninan K, Waranuch N, Sarisuta N (2011) Antioxidant activity and antiapoptotic effect of Asparagus racemosus root extracts in human lung epithelial H460 cells. Exp Ther Med 2(1):143–148 (etm-02-01-0143)
Liu CM, Sun YZ, Sun JM, Ma JQ, Cheng C (2012) Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-kappaB pathway. Biochim Biophys Acta 1820(10):1693–703 (S0304-4165(12)00188-2)
Lopez-Salas L, Borras-Linares I, Quirantes-Pine R, Emanuelli T, Segura-Carretero A, Lozano-Sanchez J (2022) Enhancing the production of the phenolic extracts of asparagus using an advanced green process. Metabolites 12(10):951. https://doi.org/10.3390/metabo12100951
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Majumdar S, Gupta S, Prajapati SK, Krishnamurthy S (2021) Neuro-nutraceutical potential of Asparagus racemosus: a review. Neurochem Int 145:105013 (S0197-0186(21)00059-0)
Mitra SK, Prakash NS, Sundaram R (2012) Shatavarins (containing Shatavarin IV) with anticancer activity from the roots of Asparagus racemosus. Indian J Pharmacol 44(6):732–6 (IJPharm-44-732)
Negi JS, Singh P, Joshi GP, Rawat MS, Bisht VK (2010) Chemical constituents of Asparagus. Pharmacogn Rev 4(8):215–20 (PRev-4-215)
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854. https://doi.org/10.1016/s0006-291x(72)80218-3
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243. https://doi.org/10.1002/tox.21994
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Plangsombat N, Rungsardthong K, Kongkaneramit L, Waranuch N, Sarisuta N (2016) Anti-inflammatory activity of liposomes of Asparagus racemosus root extracts prepared by various methods. Exp Ther Med 12(4):2790–2796 (ETM-0-0-3661)
Poormoosavi SM, Najafzadehvarzi H, Behmanesh MA, Amirgholami R (2018) Protective effects of Asparagus officinalis extract against bisphenol A- induced toxicity in Wistar rats. Toxicol Rep 5:427–433 (S2214-7500(18)30116-1)
Rong J, Chang W, Lv L, Chen J (2008) Study on the roles of nuclear factor-kappaB, p53 and Bcl-2 gene in lead acetate induced apoptosis in PC12 cells. Wei Sheng Yan Jiu 37(3):262–3268
Shao Y, Chin CK, Ho CT, Ma W, Garrison SA, Huang MT (1996) Anti-tumor activity of the crude saponins obtained from asparagus. Cancer Lett 104(1):31–6. https://doi.org/10.1016/0304383596042334
Sharma RP, Street JC, Shupe JL, Bourcier DR (1982) Accumulation and depletion of cadmium and lead in tissues and milk of lactating cows fed small amounts of these metals. J Dairy Sci 65(6):972–9. https://doi.org/10.3168/jds.S0022-0302(82)82298-4. (S0022-0302(82)82298-4 [pii])
Shi Y, Tian C, Yu X, Fang Y, Zhao X, Zhang X, et al (2020) Protective effects of Smilax glabra Roxb. Against lead-induced renal oxidative stress, inflammation and apoptosis in weaning rats and HEK-293 cells (Original Research). Front Pharmacol 11. https://doi.org/10.3389/fphar.2020.556248
Shirato K, Takanari J, Ogasawara J, Sakurai T, Imaizumi K, Ohno H et al (2016) Enzyme-treated asparagus extract attenuates hydrogen peroxide-induced matrix metalloproteinase-9 expression in murine skin fibroblast L929 cells. Nat Prod Commun 11(5):677–680
Shirato K, Koda T, Takanari J, Sakurai T, Ogasawara J, Imaizumi K et al (2018) Anti-inflammatory effect of ETAS(R)50 by inhibiting nuclear factor-kappaB p65 nuclear import in ultraviolet-B-irradiated normal human dermal fibroblasts. Evid Based Complement Alternat Med 2018:5072986. https://doi.org/10.1155/2018/5072986
Shirato K, Takanari J, Kizaki T (2021) Standardized extract of Asparagus officinalis stem attenuates SARS-CoV-2 spike protein-induced IL-6 and IL-1beta production by suppressing p44/42 MAPK and Akt phosphorylation in murine primary macrophages. Molecules 26(20):6189. https://doi.org/10.3390/molecules26206189
Singh N, Kumar A, Gupta VK, Sharma B (2018) Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem Res Toxicol 31(10):1009–1021. https://doi.org/10.1021/acs.chemrestox.8b00193
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Symes A, Shavandi A, Zhang H, Mohamed Ahmed IA, Al-Juhaimi FY, Bekhit AE (2018) Antioxidant activities and caffeic acid content in New Zealand Asparagus (Asparagus officinalis) roots extracts. Antioxidants (Basel) 7(4):52. https://doi.org/10.3390/antiox7040052
Thakur M, Thompson D, Connellan P, Deseo MA, Morris C, Dixit VK (2011) Improvement of penile erection, sperm count and seminal fructose levels in vivo and nitric oxide release in vitro by ayurvedic herbs. Andrologia 43(4):273–277. https://doi.org/10.1111/j.1439-0272.2010.01068.x
Tian ZK, Zhang YJ, Feng ZJ, Jiang H, Cheng C, Sun JM et al (2021) Nephroprotective effect of gastrodin against lead-induced oxidative stress and inflammation in mice by the GSH, Trx, Nrf2 antioxidant system, and the HMGB1 pathway. Toxicol Res (Camb) 10(2):249–263. https://doi.org/10.1093/toxres/tfab003
Vadivelan R, Gopala Krishnan R, Kannan R (2019) Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of alpha-amylase and alpha-glucosidase. J Tradit Complement Med 9(1):1–4 (S2225-4110(17)30126-8)
Wang L, Li J, Liu Z (2009) Effects of lead and/or cadmium on the oxidative damage of rat kidney cortex mitochondria. Biol Trace Elem Res. https://doi.org/10.1007/s12011-009-8560-1
Yabe J, Nakayama SM, Nakata H, Toyomaki H, Yohannes YB, Muzandu K et al (2020) Current trends of blood lead levels, distribution patterns and exposure variations among household members in Kabwe. Zambia. Chemosphere 243:125412 (S0045-6535(19)32652-9)
Yedjou CG, Tchounwou HM, Tchounwou PB (2015) DNA damage, cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int J Environ Res Public Health 13(1):ijerph13010056. https://doi.org/10.3390/ijerph13010056
Yin M, Jiang N, Guo L, Ni Z, Al-Brakati AY, Othman MS, Abdel Moneim AE, Kassab RB (2019) Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci 232:116634. https://doi.org/10.1016/j.lfs.2019.116634
Yuniarti WM, Krismaharani N, Ciptaningsih P, Celia K, Veteriananta KD, Ma’ruf A et al (2021) The protective effect of Ocimum sanctum leaf extract against lead acetate-induced nephrotoxicity and hepatotoxicity in mice (Mus musculus). Vet World 14(1):250–258 (Vetworld-14-250)
Zhang H, Birch J, **e C, Yang H, El-Din Bekhit A (2019a) Optimization of ultrasound assisted extraction method for phytochemical compounds and in-vitro antioxidant activity of New Zealand and China Asparagus cultivars (officinalis L.) roots extracts. Food Chem 294:276–284 (S0308-8146(19)30465-0)
Zhang T, Chen S, Chen L, Zhang L, Meng F, Sha S et al (2019b) Chlorogenic acid ameliorates lead-induced renal damage in mice. Biol Trace Elem Res 189(1):109–117. https://doi.org/10.1007/s12011-018-1508-6
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2023R96), King Saud University, Riyadh, Saudi Arabia.
Funding
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2023R96), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
R.A. and N.M.A. designed the project, performed the experiments and drafted and edited the manuscript. R.A. analyzed and interpreted the data and supplied the chemicals and reagents. Both authors drafted and edited the manuscript. All authors approved the final draft.
Corresponding author
Ethics declarations
Ethical approval
All the experimental protocols and procedures used in this study were approved by the Research Ethics Committee (REC) authorized the regulations and procedures governing the handling and care of animals. The animals were treated under KSU-SE-21–44 approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent statement
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almeer, R., Alyami, N.M. Renal-protective effect of Asparagus officinalis aqueous extract against lead-induced nephrotoxicity mouse model. Environ Sci Pollut Res 30, 112745–112757 (2023). https://doi.org/10.1007/s11356-023-30280-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30280-7