Abstract
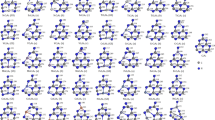
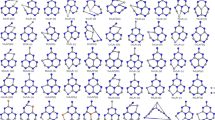
Structures, electronic, and magnetic properties of transition metal (TM) inserted W6O18 clusters have been investigated by using density functional theory. The Ti@W6O18, Ni@W6O18, Zr@W6O18, Rh@W6O18, W@W6O18, and Ir@W6O18 clusters are more structurally stable while the V@W6O18, Fe@W6O18, Zn@W6O18, Y@W6O18, Nb@W6O18, Pd@W6O18, La@W6O18, Re@W6O18, Hg@W6O18 clusters are more chemically stable. The amount of charge transfer between the TM atoms and W6O18 clusters decreases with the increase of the subgroup number except for subgroup number is equal to 11 and 12. The d orbital of the 3d TM@W6O18 clusters start to make the main contributions to Fermi level except for the Cu@W6O18 and Zn@W6O18 clusters.










Similar content being viewed by others
Data availability
The online version contains supplementary material. Final Coordinates (Angstroms) of the W6O18 and TM@W6O18 (TM = Sc ~ Zn and Re) clusters have been listed in supplementary material section.
References
Sai L, Tang L, Huang X, Chen G, Zhao J, Wang J (2012) Lowest-energy structures of (WO3)n (2≤n≤12) clusters from first-principles global search. Chem Phys Lett 544:7–12
Kruger P, Koutiri I, Bourgeois S (2012) First-principles study of hexagonal tungsten trioxide: nature of lattice distortions and effect of potassium do**. Phys Rev B 86:224102 (6 pages)
Pagnier T, Pasturel A (2003) An ab initio study of WO3 under pressure up to 30 GPa. J Phys: Condens Matter 15:3121–3133
Walkingshaw AD, Spaldin NA, Artacho E (2004) Density-functional study of charge do** in WO3, Phys Rev B 70:165110 (7 pages)
** H, Zhu J, Hu J, Li Y, Zhang Y, Huang X, Ding K, Chen W (2011) Structural and electronic properties of tungsten trioxides: from cluster to solid surface. Theor Chem Acc 130:103–114
Zhai H-J, Huang X, Cui L-F, Li X, Li J, Wang L-S (2005) Electronic and structural evolution and chemical bonding in ditungsten oxide clusters: W2On- and W2On (n=1-6). J Phys Chem A 109:6019–6030
Li Z, Fang Z, Kelley MS, Kay BD, Rousseau R, Dohnalek Z, Dixon DA (2014) Ethanol conversion on cyclic (MO3)3 (M = Mo, W) clusters. J Phys Chem C 118:4869–4877
Fujioka Y, Frantti J, Nieminen RM, Asiri AM (2013) Formation of ruthenium cluster on nanocrystalline tungsten trioxide. J Phys Chem C 117:7506–7510
Li D, Huang W-Q, **e Z, Xu L, Yang Y-C, Hu W, Huang G-F (2016) Mechanism of enhanced photocatalytic activities on tungsten trioxide doped with sulfur: dopant-type effects, Mod Phys Lett B 30:1650340 (21 pages)
Karuppasamy A (2015) Electrochromism and photocatalysis in dendrite structured Ti:WO3 thin films grown by sputtering. Appl Surf Sci 359:841–846
Karuppasamy KM, Subrahmanyam A (2008) The electrochromic and photocatalytic properties of electron beam evaporated vanadium-doped tungsten oxide thin films. Sol Energy Mater Sol Cells 92:1322–1326
Cheng XF, Leng WH, Liu DP, Zhang JQ, Cao CN (2007) Enhanced photoelectrocatalytic performance of Zn-doped WO3 photocatalysts for nitrite ions degradation under visible light. Chemosphere 68:1976–1984
Song XC, Yang E, Liu G, Zhang Y, Liu ZS, Chen HF, Wang Y (2010) Preparation and photocatalytic activity of Mo-doped WO3 nanowires. J Nanopart Res 12:2813–2819
Mal P, Bera G, Rambabu P, Turpu GR, Chakraborty B, Ramaniah LM, Singh RP, Sen P, Das P (2017) Electronic, magnetic and spectroscopic properties of doped Mn(1−x)AxWO4 (A = Co, Cu, Ni and Fe) multiferroic: an experimental and DFT study, J Phys: Condens Matter 29:075901 (10 pages)
Hameed A, Gondal MA, Yamani ZH (2004) Effect of transition metal do** on photocatalytic activity of WO3 for water splitting under laser illumination: role of 3d-orbitals. Catal Commun 5:715–719
Kim YK, Dohnalek Z, Kay BD, Rousseau R (2009) Competitive oxidation and reduction of aliphatic alcohols over (WO3)3 clusters. J Phys Chem C 113:9721–9730
Santo N, Filipescu M, Ossi PM, Dinescu M (2010) Nanostructure evolution in cluster-assembled WOx films synthesized by radio-frequency assisted laser ablation. Appl Phys A 101:325–331
Sun Q, Rao BK, Jena P, Stolcic D, Kim YD, Gantefor G, Castleman AW Jr (2004) Appearance of bulk properties in small tungsten oxide clusters. J Chem Phys 121:9417–9422
Li S, Dixon DA (2006) Molecular and electronic structures, Brönsted basicities, and Lewis acidities of group VIB transition metal oxide clusters. J Phys Chem A 110:6231–6244
Rothgeb DW, Hossain E, Kuo AT, Troyer JL, Jarrold CC (2009) Structures of MoxW(3-x)O6 (x=0–3) anion and neutral clusters determined by anion photoelectron spectroscopy and density functional theory calculations, J Chem Phys 131:044310 (14 pages)
Wang F, Valentin CD, Pacchioni G (2012) Do** of WO3 for photocatalytic water splitting: hints from density functional theory. J Phys Chem C 116:8901–8909
Xu L, Yin M-L, Liu (Frank) S (2014) Agx@WO3 core-shell nanostructure for LSP enhanced chemical sensors, Sci Rep-UK 4:6745 (7 pages)
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Li W, Da P, Zhang Y, Wang Y, Lin X, Gong X, Zheng G (2014) WO3 nanoflakes for enhanced photoelectrochemical conversion. ACS Nano 8:11770–11777
Zhao Z, Li Z, Xue G, Shen X, Wu J (2021) First-principles calculations on the structures and electronic properties of the TMn@Si24H24O36 (TM=Cu, Ag and Au, n=1–8) clusters. Mater Chem Phys 262:124272 (5 pages)
van Wüllen C (1999) Relativistic all-electron density functional calculations. J Comput Chem 20:51–62
Mulliken RS (1955) Electron population analysis on LCAO-MO molecular wave functions. II. Overlap populations, bond orders, and covalent bond energies. J Chem Phys 23:1841–1846
Cora F, Patel A, Harrison NM, Dovesi R, Catlow CRA (1996) An ab initio Hartree-Fock study of the cubic and tetragonal phases of bulk tungsten trioxide. J Am Chem Soc 118:12174–12182
Huang X, Zhai H-J, Li J, Wang L-S (2006) On the structure and chemical bonding of tri-tungsten oxide clusters W3On- and W3On (n=7-10): W3O8 as a potential molecular model for O-deficient defect sites in tungsten oxides. J Phys Chem A 110:85–92
Li Z, Shen X, Zhao Z (2022) Structures, electronic and magnetic properties of the FemOn@Cx (m=1-3, n=1-4, x=50, 60) clusters. Res Chem Intermediat 48:339–349
Li Z, Zhao Z, Shao T-T (2020) Structures, electronic and magnetic properties of transition metal atoms encapsulated in Si12C12 cage. Mod Phys Lett B 34:2050320 (10 pages)
Matxain JM, Eriksson LA, Formoso E, Piris M, Ugalde JM (2007) Endohedral (X@ZniSi)i=4-160,± nanoclusters, X = Li. Na, K, Cl, Br. J Phys Chem C 111:3560–3565
Zhai H-J, Kiran B, Cui L-F, Li X, Dixon DA, Wang L-S (2004) Electronic structure and chemical bonding in MOn- and MOn Clusters (M=Mo, W; n=3-5): a photoelectron spectroscopy and ab initio study. J Am Chem Soc 126:16134–16141
Zhao L, Qu X, Wang Y, Lv J, Zhang L, Hu Z, Gu G, Ma Y (2017) Effects of manganese do** on the structure evolution of small-sized boron clusters. J Phys: Condens Matter 29:265401 (7 pages)
Zheng H, Ou JZ, Strano MS, Kaner RB, Mitchell A, Kalantar-zadeh K (2011) Nanostructured tungsten oxide-properties, synthesis, and applications. Adv Funct Mater 21:2175–2196
Valentin CD, Wang F, Pacchioni G (2013) Tungsten oxide in catalysis and photocatalysis: hints from DFT. Top Catal 56:1404–1419
Ingham B, Hendy SC, Chong SV, Tallon JL (2005) Density-functional studies of tungsten trioxide, tungsten bronzes, and related systems. Phys Rev B 72:075109 (9 pages)
Yakovkin IN, Gutowski M (2007) Driving force for the WO3(0 0 1) surface relaxation. Surf Sci 601:1481–1488
Guo S-D (2013) Density-functional theory investigation of electronic structure, elastic properties, optical properties, and lattice dynamics of Ba2ZnWO6. Chin Phys B 22:067102 (5 pages)
** Y, Rocca D, Galli G (2013) Optical properties of tungsten trioxide from first-principles calculations. Phys Rev B 87:165203 (8 pages)
Fu H, Pan C, Zhang L, Zhu Y (2007) Synthesis, characterization and photocatalytic properties of nanosized Bi2WO6, PbWO4 and ZnWO4 catalysts. Mater Res Bull 42:696–706
Wang J, Ma L, Zhao J, Wang B, Wang G (2008) Stability and magnetic properties of transition metal atoms endohedral BnNn (n=12–28) cages. J Chem Phys 128:084306 (7 pages)
Li Y, Cai C, Zhao C, Gu Y (2018) Structure determination of (Fe3O4)n+ (n=1–3) clusters via DFT, Mod Phys Lett B 30:1650239 (8 pages)
Lu P, Wu C, Li Y, Yu Z, Cao H, Wang S (2013) Investigation on structural, electronic, and magnetic properties of Mn-doped Ga12N12 clusters. J Mater Sci 48:8552–8558
Wildberger K, Stepanyuk VS, Lang P, Zeller R, Dederichs PH (2018) Magnetic Nanostructures: 4d clusters on Ag(001). Phys Rev Lett 75:509–512
Funding
This work was supported by the National Natural Science Foundation of China (No. 51634004).
Author information
Authors and Affiliations
Contributions
Zhen Zhao and Zi-hao Wu wrote the main manuscript text and Zhi Li prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Z., Wu, Zh. & Li, Z. Structures, electronic and magnetic properties of transition metal inserted W6O18 clusters. Struct Chem 34, 1395–1403 (2023). https://doi.org/10.1007/s11224-022-02106-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02106-8