Abstract
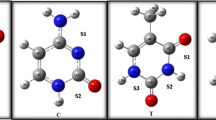
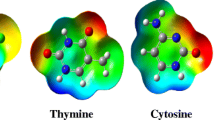
In this research work, various hydrogen-bonded complexes of 5-fluorouracil (FU), as a simplest organic anticancer drug, with the adenine and guanine purine bases were investigated. First, the molecular electrostatic potentials (MEP) of all monomers (FU, A, and G) are explored and their active sites for hydrogen-bonded interactions identified. Then, the selected plausible coplanar complexes were optimized and their complexation energies obtained. Our results reveal that the FU-G complexes are more stable than the FU-A ones. Also, the most stable structures of both series were recognized, which is consistent with the MEP results of monomers. Additionally, we estimated the strengths of the individual hydrogen bonds of the benchmark systems by energetic, geometric, spectroscopic, topological, and molecular orbital descriptors. The acceptable linear correlations between the complexation energies and some of the mentioned descriptors are observed. Finally, several aromatic indices (HOMA, ATI, NICS (0), and NICS (1)) were applied to evaluate the significant of π-electron delocalization (π-ED) of 5/6 membered rings. These results show that the π-ED of the benchmark systems increases with the formation or strengthening of the HB, which is in line with the resonance-assisted hydrogen bond theory.







Similar content being viewed by others
References
Stringer JL (2005) Basic concepts in pharmacology. McGraw-Hill Companies
Nishimura T, Okobira T, Kelly AM, Shimada N, Takeda Y, Sakurai K (2007) DNA binding of tilorone:1H NMR and calorimetric studies of the intercalation J Biochem 46:8156–8163
Aventine C, Menendes JC (2015) Medicinal chemistry of anticancer drugs. Elsevier, Amsterdam,
Braga SF, Melo LC, Barone PMVB (2004) Semiempirical study on the electronic structure of antitumor drugs ellipticines, olivacines and isoellipticines J Mol Struct THEOCHEM 710:51–59
Pang D, Abruna HD (1998) Micromethod for the investigation of the interactions between DNA and redox-active molecules J Anal Chem 70:3162–3169
Fritzsche H, Akhebat A, Taillandier E, Rippe K, Jovin TM (1993) Structure and drug interaction of parellel-stranded DNA studies by infrared spectroscope and fluorence Nucleic Acids Res 21:5085–5091
Gane PJ, Dean PM (2000) Recent advances in structure-based rational drug design J Curr Opin Struct Biol 10:401–404
Graves DE, Velea LM (2000) Intercalative binding of small molecules to nucleic acids J Curr Org Chem 4:915–929
Li VS, Choi D, Wang Z, Jimenez LS, Tang MS, Kohn H (1996) Role of the C-10 substituent in mitomycin C-1− DNA bonding J Chem Soc 118:2326–2331
Tomasz M, Lipman R, Chowdary D, Pawlak J, Verdine GL, Nakanishi K (1987) Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA Science 235:1204–1208
Hecht SM (2000) Bleomycin: new perspectives on the mechanism of action 1 J Nat Prod 63:158–168
Zuber G, Quada JC, Hecht SM (1998) Sequence selective cleavage of a DNA octanucleotide by chlorinated bithiazoles and bleomycins. J Am Chem Soc120:9368–9369
Heidelberger C, Chaudhuri NK, Danneburg P, et al. (1957) Fluorinated pyrimidines, a new class of tumour-inhibitory compounds Nature 179:663–666
Heidelberger C, Ausfield FJ (1963) Experimental and clinical use of fluorinated pyrimidines in cancer chemotherapy Cancer Res 23:1226–1243
Wanga W, Collie-Duguida E, Cassidy J (2002) FEBS Letter 531:415–420
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies Nat Rev Cancer 3:330–338
Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, Cabrera BL, Tajima A, Doctolero RT, Cabrera BL, Goel A, Arnold CA, Miyai K, Boland CR (2004) Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer Gastroenterology 126:394–401
Reni M, Cereda S, Galli L (2007) PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) for patients with advanced pancreatic cancer: the ghost regimen Cancer Lett 256:25–28
Mirzaei M (2013) Effects of carbon nanotubes on properties of the fluorouracil anticancer drug: DFT studies of a CNT-fluorouracil compound Int J Nano Dimens 3:175–179
Soltani A, Baei MT, Tazikeh Lemeski E, Kaveh AS, Balakheyli H (2015) A DFT study of 5-fluorouracil adsorption on the pure and doped BN nanotubes J Phys Chem Solids 86:57–64
Hazrati MK, Hadipour NL (2016) Adsorption behavior of 5-fluorouracil on pristine, B-, Si-, and Al-doped C60 fullerenes: a first-principles study Phys Lett A 380:937–941
Johnston PG et al. (1995) Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors Cancer Res 55:1407–1412
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven Jr T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 revision C 02 (or D 01). Gaussian Inc, Pittsburgh,
Biegler KF, Schonbohm J, Bayles D (2001) AIM2000: a program to analyze and visualize atoms in molecules J Comput Chem 22:545–559
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1992) NBO, Version 3.1 University of Wisconsin, Madison
Hameka HF (1958) On the nuclear magnetic shielding in the hydrogen molecule J Mol Phys 1:203–215
Lu T, Chen F (2012) Multiwfn: a multifunctional wave function analyzer J Comput Chem 33:580–592
Krygowski TM, Cyranski MK (1996) Separation of the energetic and geometric contributions to the aromaticity of π-electron carbocyclics Tetrahedron 52:1713–1722
Schleyer PR, Maerker C, Dransfeld A, Jiao H, Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe J Am Chem Soc 118:6317–6318
Bultinck P, Ponec R, Van Damme S (2005) Multicenter bond indices as a new measure of aromaticity in polycyclic aromatic hydrocarbons J Phys Org Chem 18:706–718
Sukhodub LF – Biofizika (1987) Interactions between nucleotide bases in coplanar and stacking dimers under vacuum. Mass spectrometric study. Eur PMC Plus 32(6): 994–1005
Scrocco E, Tomasi J (1979) Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials Adv Quantum Chem 11:115–121
Okulik N, Junert AH (2005) Theoretical analysis of the reactive sites of non-steroidal anti-inflammatory drugs Internet Electron J Mol Des 4:17–30
Politzer P, Murray J (2002) The fundamental nature and role of the electrostatic potential in atoms and molecules J Theor Chem Acc 108:134–142
Espinosa E, Molins E (2000) Retrieving interaction potentials from the topology of the electron density distribution: the case of hydrogen bonds J Chem Phys 113:5686–5694
Jesus AJL, Redinha JS (2011) Charge-assisted intramolecular hydrogen bonds in disubstituted cyclohexane derivatives J Phys Chem A 115:14069–14077
Shainyan BA, Chipanina NN, Aksamentova TN, Oznobikhina LP, Rosentsevig GN, Rosentsevig IB (2010) Intramolecular hydrogen bonds in the sulfonamide derivatives of oxamide, dithiooxamide, and biuret. FT-IR and DFT study, AIM and NBO analysis Tetrahedron 66:8551–8556
Krygowski TM, Stepion BT (2005) Sigma- and pi-electron delocalization: focus on substituent effects Chem Rev 105:3482–3512
Krygowski TM, Cyranski MK (2001) Structural aspects of aromaticity Chem Rev 101:1385–1420
Poater J, Duran M, Sola M, Silvi B (2005) Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches Chem Rev 105:3911–3947
Acknowledgements
The authors confirm that there is no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakhaei, E., Nowroozi, A. & Ravari, F. The hydrogen-bonded complexes of the 5-fluorouracil with the DNA purine bases: a comprehensive quantum chemical study. Struct Chem 29, 69–80 (2018). https://doi.org/10.1007/s11224-017-1001-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-1001-4