Abstract
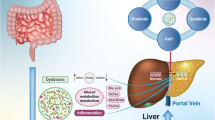
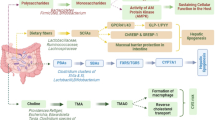
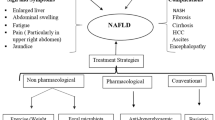
Non-alcoholic fatty liver disease (NAFLD) is by far the most prevalent form of liver disease worldwide. It’s also the leading cause of liver-related hospitalizations and deaths. Furthermore, there is a link between obesity and NAFLD risk. A projected 25% of the world’s population grieves from NAFLD, making it the most common chronic liver disorder. Several factors, such as obesity, oxidative stress, and insulin resistance, typically accompany NAFLD. Weight loss, lipid-lowering agents, thiazolidinediones, and metformin help prominently control NAFLD. Interestingly, pre-clinical studies demonstrate gut microbiota’s potential causal role in NAFLD. Increased intestinal permeability and unhindered transport of microbial metabolites into the liver are the major disruptions due to gut microbiome dysbiosis, contributing to the development of NAFLD by dysregulating the gut-liver axis. Hence, altering the pathogenic bacterial population using probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT) could benefit patients with NAFLD. Therefore, it is crucial to acknowledge the importance of microbiota-mediated therapeutic approaches for NAFLD and comprehend the underlying mechanisms that establish a connection between NAFLD and gut microbiota. This review provides a comprehensive overview of the affiliation between dysbiosis of gut microbiota and the progress of NAFLD, as well as the potential benefits of prebiotic, probiotic, synbiotic supplementation, and FMT in obese individuals with NAFLD.





Similar content being viewed by others
Data Availability
No data were used in this review study.
Abbreviations
- BA:
-
Bile acids
- DNA:
-
deoxyribonucleic acid
- FFA:
-
free fatty acid
- FMT:
-
fecal microbiota transplantation
- FXR:
-
Farnesoid X receptor
- LPS:
-
lipopolysaccharides
- mRNA:
-
Messenger ribonucleic acid
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
non-alcoholic steatohepatitis
- NLR:
-
NOD-like receptor
- NLRP3:
-
nucleotide-binding,and oligomerization domain-like receptor family pyrin domain-containing 3
- SBAs:
-
Secondary bile acids
- SCFA:
-
Short chain fatty acid
- T2DM:
-
type 2 diabetes mellitus
- TG:
-
triglycerides
- TLR:
-
Toll-like receptor
- TMAO:
-
Trimethylamine N-oxide
- TNF-α:
-
tumor necrosis factor-alpha
References
Lim S, Kim JW, Targher G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. 2021;32(7):500–14.
Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J Obes Metab Syndr. 2022;31(1):17–27.
Kuchay MS, Martínez-Montoro JI, Choudhary NS, et al. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicines. 2021;9(10):1346.
Overi D, Carpino G, Franchitto A, et al. Hepatocyte injury and hepatic stem cell niche in the progression of non-alcoholic steatohepatitis. Cells. 2020;9(3):590.
Alam S, Mustafa G, Alam M, et al. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J Gastrointest Pathophysiol. 2016;7(2):211–7.
Marušić M, Paić M, Knobloch M, et al. NAFLD, insulin resistance, and diabetes mellitus type 2. Can J Gastroenterol Hepatol. 2021;2021:1–9.
Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42.
Samuel VT, Shulman GI. Nonalcoholic fatty liver disease, insulin resistance, and ceramides. Phimister EG, ed. N Engl J Med. 2019;381(19):1866–1869.
Dharmalingam M, Yamasandhi P. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocr Metab. 2018;22(3):421.
Zhang J, Zhao Y, Xu C, et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4(1):5832.
Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399–406.
Hou K, Wu ZX, Chen XY, et al. Microbiota in health and diseases. Sig Transduct Target Ther. 2022;7(1):135.
Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol. 2020;6(1):9.
Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20(4):461–72.
Jia X, Xu W, Zhang L, et al. Impact of gut microbiota and microbiota-related metabolites on hyperlipidemia. Front Cell Infect Microbiol. 2021;11:634780.
Anand S, Mande SS. Host-microbiome interactions: gut-liver axis and its connection with other organs. NPJ Biofilms Microbiomes. 2022;8(1):89.
Jasirwan COM, Lesmana CRA, Hasan I, et al. The role of gut microbiota in non-alcoholic fatty liver disease: pathways of mechanisms. Biosci Microbiota Food Health. 2019;38(3):81–8.
Breton J, Galmiche M, Déchelotte P. Dysbiotic gut bacteria in obesity: an overview of the metabolic mechanisms and therapeutic perspectives of next-generation probiotics. Microorganisms. 2022;10(2):452.
Kang GG, Trevaskis NL, Murphy AJ, et al. Diet-induced gut dysbiosis and inflammation: key drivers of obesity-driven NASH. iScience. 2023;26(1):105905.
Asadi A, Shadab Mehr N, Mohamadi MH et al. Obesity and gut–microbiota–brain axis: a narrative review. Clin Lab Anal 2022;36(5).
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. Longo DL, ed. N Engl J Med. 2017;377(21):2063–2072.
Henao-Mejia J, Elinav E, ** C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–85.
Feng YY, Chen H. [Nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 inflammasome in Alzheimer’s disease]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2021;43(5):788–95.
Tang R, Liu R, Zha H et al. Gut microbiota induced epigenetic modifications in the non-alcoholic fatty liver disease pathogenesis. Eng Life Sci. 2023;e2300016.
Atic AI, Thiele M, Munk A, et al. Circulating miRNAs associated with nonalcoholic fatty liver disease. Am J Physiol Cell Physiol. 2023;324:C588–c602.
Arab JP, Arrese M, Shah VH. Gut microbiota in non-alcoholic fatty liver disease and alcohol‐related liver disease: current concepts and perspectives. Hepatol Res. 2020;50(4):407–18.
Hydes TJ, Ravi S, Loomba R, et al. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin Mol Hepatol. 2020;26(4):383–400.
Zelber-Sagi S. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. WJG. 2011;17(29):3377.
Park Y, Sinn DH, Kim K, et al. Associations of physical activity domains and muscle strength exercise with non-alcoholic fatty liver disease: a nation-wide cohort study. Sci Rep. 2023;13(1):4724.
Lange NF, Graf V, Caussy C, et al. PPAR-targeted therapies in the treatment of non-alcoholic fatty liver disease in diabetic patients. IJMS. 2022;23(8):4305.
Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med. 2022;292(2):190–204.
Głuszyńska P, Lemancewicz D, Dzięcioł JB, et al. Non-alcoholic fatty liver disease (NAFLD) and bariatric/metabolic surgery as its treatment option: a review. JCM. 2021;10(24):5721.
Carpi RZ, Barbalho SM, Sloan KP, et al. The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. IJMS. 2022;23(15):8805.
Khan A, Ding Z, Ishaq M, et al. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: recent updates. Int J Biol Sci. 2021;17(3):818–33. https://doi.org/10.7150/ijbs.56214.
Tan P, Li X, Shen J, et al. Fecal microbiota transplantation for the treatment of inflammatory bowel disease: an update. Front Pharmacol. 2020;11:574533.
Zheng L, Ji YY, Wen XL, et al. Fecal microbiota transplantation in the metabolic diseases: current status and perspectives. WJG. 2022;28(23):2546–60.
Zhao Y, Gong C, Xu J, et al. Research progress of fecal microbiota transplantation in liver diseases. JCM. 2023;12(4):1683.
Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–25.
Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14.
Woźniak D, Cichy W, Przysławski J, et al. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv Med Sci. 2021;66(2):284–92.
**a Y, Ren M, Yang J, et al. Gut microbiome and microbial metabolites in NAFLD and after bariatric surgery: correlation and causality. Front Microbiol. 2022;13:1003755.
Singh R, Zogg H, Wei L, et al. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J Neurogastroenterol Motil. 2021;27(1):19–34.
Abenavoli L, Scarlata GGM, Scarpellini E, et al. Metabolic-dysfunction-associated fatty liver disease and gut microbiota: from fatty liver to dysmetabolic syndrome. Medicina. 2023;59(3):594.
Hrncir T. Gut microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. 2022;10(3):578.
Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3(1):4–14.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506.
Forlano R, Mullish BH, Roberts LA, et al. The intestinal barrier and its dysfunction in patients with metabolic diseases and non-alcoholic fatty liver disease. IJMS. 2022;23(2):662.
Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: sha** our immune responses throughout life. Tissue Barriers. 2017;5(4):e1373208.
Zhou J, Tripathi M, Sinha RA et al. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. HR. 2021;2021.
Fang J, Yu CH, Li XJ, et al. Gut dysbiosis in nonalcoholic fatty liver disease: pathogenesis, diagnosis, and therapeutic implications. Front Cell Infect Microbiol. 2022;12:997018.
Pan Y, Zhang X. Diet and gut microbiome in fatty liver and its associated liver cancer. J Gastro and Hepatol. 2022;37(1):7–14.
Jiang X, Zheng J, Zhang S, et al. Advances in the involvement of gut microbiota in pathophysiology of NAFLD. Front Med. 2020;7:361.
Albhaisi SAM, Bajaj JS. The influence of the microbiome on NAFLD and NASH. Clin Liver Dis. 2021;17(1):15–8.
Gudan A, Kozłowska-Petriczko K, Wunsch E, et al. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease: what do we know in 2023? Nutrients. 2023;15(6):1323.
Deehan EC, Zhang Z, Riva A, et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome. 2022;10(1):77.
Wang H, Mehal W, Nagy LE, et al. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell Mol Immunol. 2021;18(1):73–91.
Fukunishi S, Sujishi T, Takeshita A, et al. Lipopolysaccharides accelerate hepatic steatosis in the development of nonalcoholic fatty liver disease in Zucker rats. J Clin Biochem Nutr. 2014;54(1):39–44.
Staley C, Weingarden AR, Khoruts A, et al. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101(1):47–64.
Bertolini A, Fiorotto R, Strazzabosco M. Bile acids and their receptors: modulators and therapeutic targets in liver inflammation. Semin Immunopathol. 2022;44(4):547–64.
Stofan M, Guo GL. Bile acids and fxr: novel targets for liver diseases. Front Med. 2020;7:544.
Kiriyama Y, Nochi H. Physiological role of bile acids modified by the gut microbiome. Microorganisms. 2021;10(1):68.
Yu Y, Raka F, Adeli K. The role of the gut microbiota in lipid and lipoprotein metabolism. JCM. 2019;8(12):2227.
Radun R, Trauner M. Role of FXR in bile acid and metabolic homeostasis in NASH: pathogenetic concepts and therapeutic opportunities. Semin Liver Dis. 2021;41(04):461–75.
https://www.statnews.com/2023/06/22/intercept-nash-obeticholic-acid-fda-rejection.
Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22.
Xu Z, Jiang W, Huang W, et al. Gut microbiota in patients with obesity and metabolic disorders — a systematic review. Genes Nutr. 2022;17(1):2.
Liwinski T, Heinemann M, Schramm C. The intestinal and biliary microbiome in autoimmune liver disease-current evidence and concepts. Semin Immunopathol. 2022;44(4):485–507.
Li F, Ye J, Shao C, et al. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: a systematic review and Meta-analysis. Lipids Health Dis. 2021;20(1):22.
Juárez-Fernández M, Porras D, Petrov P, et al. The synbiotic combination of Akkermansia Muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota resha** and bile acid metabolism modulation. Antioxidants. 2021;10(12):2001.
Xu Y, Wang N, Tan HY, et al. Function of akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219.
Régnier M, Rastelli M, Morissette A, et al. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansia Muciniphila in mice. Nutrients. 2020;12(10):2932.
Gieryńska M, Szulc-Dąbrowska L, Struzik J, et al. Integrity of the intestinal barrier: the involvement of epithelial cells and microbiota—a mutual relationship. Animals. 2022;12(2):145.
Mori–Akiyama Y, Van Den Born M, Van Es JH, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133(2):539–46.
Zhang S, Tun HM, Zhang D, et al. Alleviation of hepatic steatosis: dithizone-related gut microbiome restoration during paneth cell dysfunction. Front Microbiol. 2022;13:813783.
Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–43.
Yang S, Yu M. Role of goblet cells in intestinal barrier and mucosal immunity. JIR 2021;Volume 14:3171–83.
Fang J, Wang H, Zhou Y, et al. Slimy partners: the mucus barrier and gut microbiome in ulcerative colitis. Exp Mol Med. 2021;53(5):772–87.
Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017;358(1):39–44.
Yuksel H, Ocalan M, Yilmaz O. E-cadherin: an important functional molecule at respiratory barrier between defence and dysfunction. Front Physiol. 2021;12:720227.
Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10(1):a029314.
Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal barrier in human health and disease. IJERPH. 2021;18(23):12836.
**ong J, Chen X, Zhao Z, et al. A potential link between plasma short-chain fatty acids, TNF-α level and disease progression in non-alcoholic fatty liver disease: a retrospective study. Exp Ther Med. 2022;24(3):598.
Sohail MU, Althani A, Anwar H et al. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res 2017: 9631435.
Mallat A, Teixeira-Clerc F, Deveaux V, et al. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. Br J Pharmacol. 2011;163(7):1432–40.
Srivastava RK, Lutz B, Ruiz de Azua I. The Microbiome and gut endocannabinoid system in the regulation of stress responses and metabolism. Front Cell Neurosci. 2022;16:867267.
Arias N, Arboleya S, Allison J, et al. The relationship between Choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. 2020;12(8):2340.
Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91.
Khan A, Ding Z, Ishaq M, et al. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: recent updates. Int J Biol Sci. 2021;17(3):818–33.
Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021.
Luo M, Yan J, Wu L, et al. Probiotics alleviated nonalcoholic fatty liver disease in high-fat diet-fed rats via gut microbiota/fxr/fgf15 signaling pathway. J Immunol Res. 2021;2021:1–10.
Huang Y, Wang X, Zhang L, et al. Effect of probiotics therapy on nonalcoholic fatty liver disease. Comput Math Methods Med. 2022;2022:1–15.
Arai N, Miura K, Aizawa K, et al. Probiotics suppress nonalcoholic steatohepatitis and carcinogenesis progression in hepatocyte-specific PTEN knockout mice. Sci Rep. 2022;12(1):16206.
Ritze Y, Bárdos G, Claus A et al. Lactobacillus rhamnosus gg protects against non-alcoholic fatty liver disease in mice. Covasa M, ed. PLoS ONE. 2014;9(1):e80169.
Scorletti E, Afolabi PR, Miles EA, et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. 2020;158(6):1597–1610e7.
Kobyliak N, Abenavoli L, Mykhalchyshyn G, et al. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in nafld patients: evidence from a randomized clinical trial. JGLD. 2018;27(1):41–9.
Behrouz V, Aryaeian N, Zahedi MJ, et al. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: a randomized clinical trial. J Food Sci. 2020;85(10):3611–7.
Chong CYL, Orr D, Plank LD, et al. Randomised double-blind placebo-controlled trial of inulin with metronidazole in non-alcoholic fatty liver disease (NAFLD). Nutrients. 2020;12(4):937.
Ayob N, Muhammad Nawawi KN, Mohamad Nor MH, et al. The effects of probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines. 2023;11(2):640.
Naudin CR, Maner-Smith K, Owens JA, et al. Lactococcus lactis subspecies cremoris elicits protection against metabolic changes induced by a western-style diet. Gastroenterology. 2020;159(2):639–651e5.
Choi SI, You S, Kim S et al. Weissella cibaria MG5285 and Lactobacillus reuteri MG5149 attenuated fat accumulation in adipose and hepatic steatosis in high-fat diet-induced C57BL/6J obese mice. Food & Nutr Res. 2021;65.
Werlinger P, Nguyen HT, Gu M, et al. Lactobacillus reuteri mjm60668 prevent progression of non-alcoholic fatty liver disease through anti-adipogenesis and anti-inflammatory pathway. Microorganisms. 2022;10(11):2203.
Hu W, Gao W, Liu Z, et al. Specific strains of faecalibacterium prausnitzii ameliorate nonalcoholic fatty liver disease in mice in association with gut microbiota regulation. Nutrients. 2022;14(14):2945.
Bakhshimoghaddam F, Shateri K, Sina M, et al. Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr. 2018;148(8):1276–84.
Ahn SB, Jun DW, Kang BK, et al. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep. 2019;9(1):5688.
Chong PL, Laight D, Aspinall RJ, et al. A randomised placebo controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. 2021;21(1):144.
Nguyen HT, Gu M, Werlinger P, et al. Lactobacillus sakei mjm60958 as a potential probiotic alleviated non-alcoholic fatty liver disease in mice fed a high-fat diet by modulating lipid metabolism, inflammation, and gut microbiota. IJMS. 2022;23(21):13436.
Do MH, Oh MJ, Lee HB, et al. Bifidobacterium animalis ssp. Lactis mg741 reduces body weight and ameliorates nonalcoholic fatty liver disease via improving the gut permeability and amelioration of inflammatory cytokines. Nutrients. 2022;14(9):1965.
Yan Y, Liu C, Zhao S, et al. Probiotic Bifidobacterium lactis V9 attenuates hepatic steatosis and inflammation in rats with non-alcoholic fatty liver disease. AMB Expr. 2020;10(1):101.
Zhang Z, Zhou H, Zhou X, et al. lactobacillus casei yrl577 ameliorates markers of non-alcoholic fatty liver and alters expression of genes within the intestinal bile acid pathway. Br J Nutr. 2021;125(5):521–9.
Kothari D, Patel S, Kim SK. Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother. 2019;111:537–47.
Kim S, Lee Y, Kim Y, et al. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. 2020;86:e03004–19.
Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–13.
Jian H, Liu Y, Wang X, et al. Akkermansia muciniphila as a next-generation probiotic in modulating human metabolic homeostasis and disease progression: a role mediated by gut-liver-brain axes? Int J Mol Sci. 2023;24(4):3900.
Chiantera V, Laganà AS, Basciani S, et al. A critical perspective on the supplementation of Akkermansia muciniphila: benefits and harms. Life (Basel). 2023;13(6):1247.
Zou Y, Chen T. Engineered Akkermansia muciniphila: a promising agent against diseases (review). Exp Ther Med. 2020;20(6):285.
Han TR, Yang WJ, Tan QH, et al. Gut microbiota therapy for nonalcoholic fatty liver disease: evidence from randomized clinical trials. Front Microbiol. 2023;13:1004911.
Cho JM, Pestana L, Pardi R, et al. Fecal microbiota transplant via colonoscopy may be preferred due to intraprocedure findings. Intest Res. 2019;17(3):434–7.
Almeida C, Oliveira R, Baylina P, et al. Current trends and challenges of fecal microbiota transplantation—an easy method that works for all? Biomedicines. 2022;10(11):2742.
Xue L, Deng Z, Luo W, et al. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol. 2022;12:759306.
Witjes JJ, Smits LP, Pekmez CT, et al. Donor fecal microbiota transplantation alters gut microbiota and metabolites in obese individuals with steatohepatitis. Hepatol Commun. 2020;4(11):1578–90.
Craven L, Rahman A, Nair Parvathy S, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. 2020;115:1055–65.
Lahtinen P, Juuti A, Luostarinen M, et al. Effectiveness of fecal microbiota transplantation for weight loss in patients with obesity undergoing bariatric surgery: a randomized clinical trial. JAMA Netw Open. 2022;5(12):e2247226.
Zhou D, Pan Q, Shen F, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7(1):1529.
Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916e7.
Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–71.
Freitag TL, Hartikainen A, Jouhten H, et al. Minor effect of antibiotic pre-treatment on the engraftment of donor microbiota in fecal transplantation in mice. Front Microbiol. 2019;10:2685.
Bárcena C, Valdés-Mas R, Mayoral P, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25(8):1234–42.
Le Bastard Q, Ward T, Sidiropoulos D, et al. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep. 2018;8(1):6219.
Zeng X, Li X, Li X, et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood. 2023;141(14):1691–707.
Park SY, Seo GS. Fecal microbiota transplantation: is it safe? Clin Endosc. 2021;54(2):157–60.
Forlano R, Sivakumar M, Mullish BH, et al. Gut microbiota—a future therapeutic target for people with non-alcoholic fatty liver disease: a systematic review. IJMS. 2022;23(15):8307.
Pandey Kavita R, Naik Suresh R, Vakil Babu V. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015;52(12):7577–87.
Marrs T, Walter J. Pros and cons: is faecal microbiota transplantation a safe and efficient treatment option for gut dysbiosis? Allergy. 2021;76(7):2312–7.
Kolodziejczyk AA, Zheng D, Shibolet O, et al. The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11(2):e9302.
Tkach S, Dorofeyev A, Kuzenko I, et al. Current status and future therapeutic options for fecal microbiota transplantation. Med (Kaunas). 2022;58(1):84.
Acknowledgements
The authors thank the Tenth People’s Affiliated Hospital of Tongji University for providing the workplace to prepare the review.
Funding
This work was supported by the National Key R&D Program of China (No. 2018YFC1314101, 2016YFC1305600), National Natural Science Foundation of China (82170861, 81970677), Fundamental Research Funds for the Central Universities of Tongji University (22120190210), Clinical Research Plan of SHDC(SHDC2020CR1017B), and Shanghai Committee of Science and Technology, China (19DZ1910200, 18411951803, 17DZ1910603).
Author information
Authors and Affiliations
Contributions
Muthukumaran Jayachandran has conceptualized, written, proofread, and finalized the review; Qu Shen has conceptualized, proofread and approved the study.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jayachandran, M., Qu, S. Non-alcoholic fatty liver disease and gut microbial dysbiosis- underlying mechanisms and gut microbiota mediated treatment strategies. Rev Endocr Metab Disord 24, 1189–1204 (2023). https://doi.org/10.1007/s11154-023-09843-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09843-z