Abstract
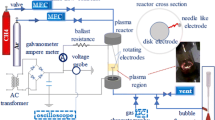
The environmental impact of greenhouse gases such as carbon dioxide and methane can be reduced if they are used as feedstock to synthesize chemical building blocks such as syngas (CO, H2) via dry reforming. Methane dry reforming is investigated using an Ar/CO2/CH4 rotating gliding arc (RGA) reactor powered by a dual-stage pulsed DC power supply. Tangential gas injection combined with a static magnetic field enabled the rotation and upward displacement of the arc along the conical cathode and the grounded anode, yielding to a larger plasma volume. Different parameters such as peak arc current (0.74 and 1.50 A), total gas flow rate (3.7, 4.7 and 6.7 SLPM), CO2/CH4 ratio (1.0, 1.5, 2.0) and gas inlet preheating (room temperature, 200 °C) were studied to determine the most efficient parameter combination. Gas conversion was measured online using a calibrated mass spectrometer and offline using a gas chromatograph. Noticeable increases in CO2 and CH4 conversions, as well as H2 and CO yields, were obtained when doubling the peak arc current. For the larger peak current, higher H2 yields were obtained at a CO2/CH4 = 1.0, and the best energy efficiencies were obtained at the lowest specific energy input values. No significant effect of the gas inlet temperature on the conversions or yields was found. Trace amounts of acetylene and ethylene, as well as some carbon deposits were observed as by-products of syngas generation. The low amount of by-products obtained implies a good selectivity for CO and H2, i.e., a cleaner syngas when produced with RGA discharge.
















Similar content being viewed by others
References
Pakhare D, Spivey J, Zhao H et al (2014) A review of dry (CO2) reforming of methane over noble metal catalysts. Chem Soc Rev 43:7813–7837. https://doi.org/10.1039/C3CS60395D
Maqueo PDG, Coulombe S, Bergthorson JM (2019) Energy efficiency of a nanosecond repetitively pulsed discharge for methane reforming. J Phys D Appl Phys. https://doi.org/10.1088/1361-6463/ab199b
North American Power Plant Air Emissions (2011). Montreal, Quebec, Canada
NASA: Global Climate Change (2018) Climate change: how do we know? https://climate.nasa.gov/evidence/. Accessed 28 Jun 2018
National Academy of Sciences (2014) Climate change: evidence and causes. National Academies Press, Washington
Lavoie J-M (2014) Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front Chem 2:81. https://doi.org/10.3389/fchem.2014.00081
Tu X, Whitehead JC (2014) Plasma dry reforming of methane in an atmospheric pressure AC gliding arc discharge: co-generation of syngas and carbon nanomaterials. Int J Hydrog Energy 39:9658–9669. https://doi.org/10.1016/j.ijhydene.2014.04.073
Tan Z, Ai P (2017) CO2 reforming of biogas to obtain synthesis gas using non-thermal plasma. J Energy Inst 90:864–874. https://doi.org/10.1016/J.JOEI.2016.08.008
Olsbye U, Wurzel T, Mleczko L (1997) Kinetic and reaction engineering studies of dry reforming of methane over a Ni/La/Al2O3 catalyst. Ind Eng Chem Res 35:5180–5188. https://doi.org/10.1021/ie970246l
Liu J-L, Park H-W, Chung W-J, Park D-W (2016) High-efficient conversion of CO2 in AC-pulsed tornado gliding arc plasma. Plasma Chem Plasma Process 36:437–449. https://doi.org/10.1007/s11090-015-9649-2
Chen G, Georgieva V, Godfroid T et al (2016) Plasma assisted catalytic decomposition of CO2. Appl Catal B Environ 190:115–124. https://doi.org/10.1016/j.apcatb.2016.03.009
Zhou LM, Xue B, Kogelschatz U, Eliasson B (1998) Nonequilibrium plasma reforming of greenhouse gases to synthesis gas. Energy Fuels 12:1191–1199. https://doi.org/10.1021/ef980044h
Brock SL, Shimojo T, Suib SL et al (2002) Application of non-thermal atmospheric pressure ac plasmas to the carbon dioxide reforming of methane. Res Chem Intermed 28:13–24. https://doi.org/10.1163/156856702760129465
Tu X, Gallon HJ, Whitehead JC (2013) Plasma-assisted reduction of a NiO/Al2O3 catalyst in atmospheric pressure H2/Ar dielectric barrier discharge. Catal Today 211:120–125. https://doi.org/10.1016/j.cattod.2013.03.024
Wang Q, Yan B-H, ** Y, Cheng Y (2009) Investigation of dry reforming of methane in a dielectric barrier discharge reactor. Plasma Chem Plasma Process 29:217–228. https://doi.org/10.1007/s11090-009-9173-3
Tu X, Whitehead JC (2012) Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: understanding the synergistic effect at low temperature. Appl Catal B Environ 125:439–448. https://doi.org/10.1016/j.apcatb.2012.06.006
Li D, Li X, Bai M et al (2009) CO2 reforming of CH4 by atmospheric pressure glow discharge plasma: a high conversion ability. Int J Hydrog Energy 34:308–313. https://doi.org/10.1016/j.ijhydene.2008.10.053
Cheng D-G, Zhu X, Ben Y et al (2006) Carbon dioxide reforming of methane over Ni/Al2O3 treated with glow discharge plasma. Catal Today 115:205–210. https://doi.org/10.1016/j.cattod.2006.02.063
Aziznia A, Bozorgzadeh HR, Seyed-Matin N et al (2012) Comparison of dry reforming of methane in low temperature hybrid plasma-catalytic corona with thermal catalytic reactor over Ni/γ-Al2O3. J Nat Gas Chem 21:466–475. https://doi.org/10.1016/S1003-9953(11)60392-7
Li M-W, Xu G-H, Tian Y-L et al (2004) Carbon dioxide reforming of methane using DC corona discharge plasma reaction. J Phys Chem A 108:1687–1693. https://doi.org/10.1021/jp037008q
Chen Q, Yang X, Sun J et al (2017) Pyrolysis and oxidation of methane in a RF plasma reactor. Plasma Chem Plasma Process 37:1551–1571. https://doi.org/10.1007/s11090-017-9844-4
Pacheco J, Soria G, Valdivia R et al (2014) Warm plasma reactor with vortex effect enhanced used for CH4–CO2 reforming. IEEE Trans Plasma Sci 42:2800–2801. https://doi.org/10.1109/TPS.2014.2337290
Sreethawong T, Thakonpatthanakun P, Chavadej S (2007) Partial oxidation of methane with air for synthesis gas production in a multistage gliding arc discharge system. Int J Hydrog Energy 32:1067–1079. https://doi.org/10.1016/j.ijhydene.2006.07.013
Indarto A, Choi J-W, Lee H, Song HK (2006) Effect of additive gases on methane conversion using gliding arc discharge. Energy 31:2986–2995. https://doi.org/10.1016/j.energy.2005.10.034
Wu A, Yan J, Zhang H et al (2014) Study of the dry methane reforming process using a rotating gliding arc reactor. Int J Hydrog Energy 39:17656–17670. https://doi.org/10.1016/J.IJHYDENE.2014.08.036
Li K, Liu J-L, Li X-S et al (2016) Warm plasma catalytic reforming of biogas in a heat-insulated reactor: dramatic energy efficiency and catalyst auto-reduction. Chem Eng J 288:671–679. https://doi.org/10.1016/j.cej.2015.12.036
Zhang H, Du C, Wu A et al (2014) Rotating gliding arc assisted methane decomposition in nitrogen for hydrogen production. Int J Hydrog Energy 39:12620–12635. https://doi.org/10.1016/J.IJHYDENE.2014.06.047
Chen HL, Lee HM, Chen SH et al (2008) Review of plasma catalysis on hydrocarbon reforming for hydrogen production—interaction, integration, and prospects. Appl Catal B Environ 85:1–9. https://doi.org/10.1016/j.apcatb.2008.06.021
Chung W-C, Chang M-B (2016) Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew Sustain Energy Rev 62:13–31. https://doi.org/10.1016/j.rser.2016.04.007
Pacheco J, Soria G, Pacheco M et al (2015) Greenhouse gas treatment and H2 production, by warm plasma reforming. Int J Hydrog Energy 40:17165–17171. https://doi.org/10.1016/j.ijhydene.2015.08.062
Neyts EC (2016) Plasma-surface interactions in plasma catalysis. Plasma Chem Plasma Process 36:185–212. https://doi.org/10.1007/s11090-015-9662-5
Zhang H, Li L, Li X et al (2018) Warm plasma activation of CO2 in a rotating gliding arc discharge reactor. J CO2 Util 27:472–479. https://doi.org/10.1016/J.JCOU.2018.08.020
Fridman A, Gutsol A, Gangoli S et al (2008) Characteristics of gliding arc and its application in combustion enhancement. J Propuls Power 24:1216–1227. https://doi.org/10.2514/1.24795
Fridman A, Nester S, Kennedy LA et al (1999) Gliding arc gas discharge. Prog Energy Combust Sci 25:211–231. https://doi.org/10.1016/S0360-1285(98)00021-5
Zhang H, Li XD, Zhang YQ et al (2012) Rotating gliding arc codriven by magnetic field and tangential flow. IEEE Trans Plasma Sci 40:3493–3498. https://doi.org/10.1109/TPS.2012.2220984
Hwang N, Lee J, Lee DH, Song Y-H (2012) Interactive phenomena of a rotating arc and a premixed CH4 flame. Plasma Chem Plasma Process 32:187–200. https://doi.org/10.1007/s11090-012-9349-0
McNall M, Coulombe S (2018) Characterization of a rotating gliding arc in argon at atmospheric pressure. J Phys D Appl Phys. https://doi.org/10.1088/1361-6463/aade44
Lesueur H, Czernichowski A, Chapelle J (1994) Electrically assisted partial oxidation of methane. Int J Hydrog Energy 19:139–144. https://doi.org/10.1016/0360-3199(94)90118-X
Nikoo MK, Amin NAS (2011) Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process Technol 92:678–691. https://doi.org/10.1016/j.fuproc.2010.11.027
Bo Z, Yan J, Li X et al (2008) Plasma assisted dry methane reforming using gliding arc gas discharge: effect of feed gases proportion. Int J Hydrog Energy 33:5545–5553. https://doi.org/10.1016/j.ijhydene.2008.05.101
Lu N, Sun D, **a Y et al (2018) Dry reforming of CH4-CO2 in AC rotating gliding arc discharge: effect of electrode structure and gas parameters. Int J Hydrog Energy 43:13098–13109. https://doi.org/10.1016/J.IJHYDENE.2018.05.053
Istadi ANAS (2006) Co-generation of synthesis gas and C2+ hydrocarbons from methane and carbon dioxide in a hybrid catalytic-plasma reactor: a review. Fuel 85:577–592. https://doi.org/10.1016/j.fuel.2005.09.002
Bogaerts A, Berthelot A, Heijkers S et al (2017) CO2 conversion by plasma technology: insights from modeling the plasma chemistry and plasma reactor design. Plasma Sources Sci Technol 26:063001
Czernichowski A (2001) Glidarc assisted preparation of the synthesis gas from natural and waste hydrocarbons gases. Oil Gas Sci Technol 56:181–198. https://doi.org/10.2516/ogst:2001018
Liu C-J, Xu G-H, Wang T (1999) Non-thermal plasma approaches in CO utilization. Fuel Process Technol 58:119–134
Sun SR, Wang HX, Mei DH et al (2017) CO2 conversion in a gliding arc plasma: Performance improvement based on chemical reaction modeling. J CO2 Util 17:220–234. https://doi.org/10.1016/j.jcou.2016.12.009
Sudhakaran MSP, Trinh HQ, Karuppiah J et al (2017) Plasma catalytic removal of p-xylene from air stream using γ-Al2O3 supported manganese catalyst. Top Catal 60:944–954. https://doi.org/10.1007/s11244-017-0759-3
Hammer T, Kappes T, Baldauf M (2004) Plasma catalytic hybrid processes: gas discharge initiation and plasma activation of catalytic processes. Catal Today 89:5–14. https://doi.org/10.1016/j.cattod.2003.11.001
Eliasson B, Liu C-J, Kogelschatz U et al (2000) Direct conversion of methane and carbon dioxide to higher hydrocarbons using catalytic dielectric-barrier discharges with zeolites. Ind Eng Chem Res. https://doi.org/10.1021/ie990804r
Ghorbanzadeh AM, Lotfalipour R, Rezaei S (2009) Carbon dioxide reforming of methane at near room temperature in low energy pulsed plasma. Int J Hydrog Energy 34:293–298. https://doi.org/10.1016/j.ijhydene.2008.10.056
Scapinello M, Martini LM, Dilecce G, Tosi P (2016) Conversion of CH4/CO2 by a nanosecond repetitively pulsed discharge. J Phys D Appl Phys. https://doi.org/10.1088/0022-3727/49/7/075602
Cleiren E, Heijkers S, Ramakers M, Bogaerts A (2017) Dry reforming of methane in a gliding arc plasmatron: towards a better understanding of the plasma chemistry. Chemsuschem 10:4025–4036. https://doi.org/10.1002/cssc.201701274
Kraus M, Egli W, Haffner K et al (2002) Investigation of mechanistic aspects of the catalytic CO2 reforming of methane in a dielectric-barrier discharge using optical emission spectroscopy and kinetic modeling. Phys Chem Chem Phys 4:668–675. https://doi.org/10.1039/b108040g
Ray D, Manoj P, Reddy K et al (2017) Ni-Mn/γ-Al2O3 assisted plasma dry reforming of methane. Catal Today 309:212–218. https://doi.org/10.1016/j.cattod.2017.07.003
Ray D, Subrahmanyam C (2016) CO2 decomposition in a packed DBD plasma reactor: influence of packing materials. RSC Adv 6:39492–39499. https://doi.org/10.1039/C5RA27085E
Fazekas P, Keszler AM, Bódis E et al (2015) Optical emission spectra analysis of thermal plasma treatment of poly(vinyl chloride). Open Chem 13:549–556. https://doi.org/10.1515/chem-2015-0069
Kopyscinski J (2010) Production of synthetic natural gas in a fluidized bed reactor. ETH Zurich, Zürich
Hodkiewicz J (2010) Characterizing carbon materials with Raman spectroscopy. Madison,WI, USA
Acknowledgments
J. Martin-del-Campo acknowledges the financial support of CONACyT and the Faculty of Engineering through the McGill Engineering Doctoral Award. The authors acknowledge the contributions of Mitchell McNall in the construction of the RGA, Elmira Pajootan for conducting the SEM analysis and Marianna Uceda for conducting the Raman Spectroscopy. The authors thank the technical staff from the Department of Chemical Engineering of McGill University, especially Luciano Cusmich, Gerald Lepkyj, and Frank Caporuscio for their assistance. This work was financially supported by the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation, and the Gerald Hatch Faculty Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martin-del-Campo, J., Coulombe, S. & Kopyscinski, J. Influence of Operating Parameters on Plasma-Assisted Dry Reforming of Methane in a Rotating Gliding Arc Reactor. Plasma Chem Plasma Process 40, 857–881 (2020). https://doi.org/10.1007/s11090-020-10074-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10074-2