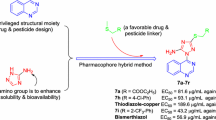
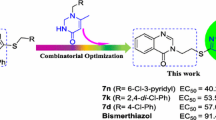
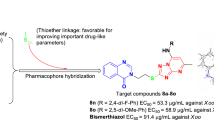
Abstract
In this paper, a series of novel 1,2,4-trizaole-substituted 1,3,4-oxadiazole derivatives with a dual thioether moiety were constructed. The synthetic compounds were characterized by 1H NMR, 13C NMR, HRMS, and single crystal diffraction. The antimicrobial activities of title compounds against fungi (Pyricutaria oryzae Cav., Phomopsis sp., Botryosphaeria dothidea, cucumber Botrytis cinerea, tobacco Botrytis cinerea, blueberry Botrytis cinerea) and bacteria (Xanthomonas oryzae pv. oryzicola, Xoc; Xanthomonas axonopodis pv. citri, Xac) revealed these compounds possessed excellent antibacterial activity through mycelial growth rate method and turbidity method, respectively. Among them, compounds 7a, 7d, 7g, 7k, 7l, and 7n had the antibacterial inhibition rate of 90.68, 97.86, 93.61, 97.70, 97.26, and 92.34%, respectively. The EC50 values of 7a, 7d, 7g, 7k, 7l, and 7n were 58.31, 48.76, 58.50, 40.11, 38.15, and 46.99 μg/mL, separately, superior to that of positive control pesticide thiodiazole copper (104.26 μg/mL). The molecular docking simulation of compound 7l and glutathione s-transferase also confirmed its good activity. The in vivo bioassay toward Xac infected citrus leaves was also performed to evaluate the potential of compounds as efficient antibacterial reagent. Further study of antibacterial mechanism was also carried out, including extracellular polysaccharide production, permeability of bacterial membrane, and scanning electron microscope observations. The excellent antibacterial activities of these compounds provided a strong support for its application for preventing and control plant diseases.
Graphical abstract
A series of novel 1,2,4-trizaole-substituted 1,3,4-oxadiazole derivatives were constructed as potential protective reagents against Xanthomonas axonopodispv. citri, Xac.









Similar content being viewed by others
References
El-Sheikh ESA, Ramadan MM, El-Sobki AE, Shalaby AA, McCoy MR, Hamed IA, Ashour MB, Hammock BD (2022) Pesticide residues in vegetables and fruits from farmer markets and associated dietary risks. Molecules 27:8072. https://doi.org/10.3390/molecules27228072
Zhang C, Yang J, Zhao C, Li L, Wu Z (2023) Potential fungicide candidates: a dual action mode study of novel pyrazole-4-carboxamides against Gibberella zeae. J Agric Food Chem 71:1862–1872. https://doi.org/10.1021/acs.jafc.2c06962
Carvalho FP (2017) Pesticides, environment, and food safety. Food Energy Secur 6:48–60. https://doi.org/10.1002/fes3.108
Afshari M, Karimi-Shahanjarini A, Khoshravesh S, Besharati F (2021) Effectiveness of interventions to promote pesticide safety and reduce pesticide exposure in agricultural health studies: a systematic review. PLoS ONE 16:e0245766. https://doi.org/10.1371/journal.pone.0245766
Xu L, El-Aty AMA, Eun JB, Shim JH, Zhao J, Lei X, Gao S, She Y, ** F, Wang J, ** M, Hammock BD (2022) Recent advances in rapid detection techniques for pesticide residue: a review. J Agric Food Chem 70:13093–13117. https://doi.org/10.1021/acs.jafc.2c05284
Malla MA, Dubey A, Raj A, Kumar A, Upadhyay N, Yadav S (2022) Emerging frontiers in microbe-mediated pesticide remediation: unveiling role of omics and in silico approaches in engineered environment. Environ Pollut 299:118851. https://doi.org/10.1016/j.envpol.2022.118851
Haider FU, Wang X, Zulfiqar U, Farooq M, Hussain S, Mehmood T, Naveed M, Li Y, Cai L, Saeed Q, Ahmad I, Mustafa A (2022) Biochar application for remediation of organic toxic pollutants in contaminated soils; an update. Ecotoxicol Environ Saf 248:114322. https://doi.org/10.1016/j.ecoenv.2022.114322
Karimi H, Mahdavi S, Lajayer BA, Moghiseh E, Rajput VD, Minkina T, Astatkie T (2022) Insights on the bioremediation technologies for pesticide-contaminated soils. Environ Geochem Health 44:1329–1354. https://doi.org/10.1007/s10653-021-01081-z
Alexandrino DAM, Almeida CMR, Mucha AP, Carvalho MF (2022) Revisiting pesticide pollution: the case of fluorinated pesticides. Environ Pollut 292:118315. https://doi.org/10.1016/j.envpol.2021.118315
Zeng D, Wang MW, **ang M, Liu LW, Wang PY, Li Z, Yang S (2020) Design, synthesis, and antimicrobial behavior of novel oxadiazoles containing various N-containing heterocyclic pendants. Pest Manag Sci 76:2681–2692. https://doi.org/10.1002/ps.5814
Li H, Liu H, Zhang Y, Yang N, **ong L, Li Z, Wang B (2021) Synthesis, insecticidal activities, and SAR studies of novel piperazine-containing heterocyclic mono-/di-/tri-amide derivatives. Chin Chem Lett 32:2893–2898. https://doi.org/10.1016/j.cclet.2021.02.002
Yuan Q, Zhang Y, Du S, Fu W, Xu Z, Cheng J, Li Z, Shao X (2023) Design, synthesis, and biological activity of heterocyclic methyl esters. J Heterocyclic Chem 60:781–791. https://doi.org/10.1002/jhet.4626
Lou J, Wang H, Wang S, Han J, Wang M (2022) Synthesis, antimicrobial activity and 3D-QSAR study of novel 5-substituted-1,3,4-thiadiazole Schiff base derivatives. J Mol Struct 1267:133629. https://doi.org/10.1016/j.molstruc.2022.133629
Chen X, Dong F, Xu J, Liu X, Wu X, Zheng Y (2016) Effective monitoring of fluxapyroxad and its three biologically active metabolites in vegetables, fruits, and cereals by optimized QuEChERS treatment based on UPLC-MS/MS. J Agric Food Chem 64:8935–8943. https://doi.org/10.1021/acs.jafc.6b03253
Gao Y, Liu Y, He L, Zhu J, Wu B, Liu F, Mu W (2021) Activity of the novel fungicide mefentrifluconazole against Colletotrichum scovillei. Plant Dis 105:1522–1530. https://doi.org/10.1094/PDIS-10-20-2157-RE
Jiang L, Wang H, Xu H, Qiao K, **a X, Wang K (2015) Transportation behaviour of fluopicolide and its control effect against Phytophthora capsici in greenhouse tomatoes after soil application. Pest Manag Sci 71:1008–1014. https://doi.org/10.1002/ps.3879
Zhu X, Zhang M, Liu J, Ge J, Yang G (2015) Ametoctradin is a potent Qo site inhibitor of the mitochondrial respiration complex III. J Agric Food Chem 63:3377–3386. https://doi.org/10.1021/acs.jafc.5b00228
Yu X, Armstrong CM, Zhou M, Duan Y (2016) Bismerthiazol inhibits Xanthomonas citri subsp. citri growth and induces differential expression of citrus defense-related genes. Phytopathology 106:693–701. https://doi.org/10.1094/PHYTO-12-15-0328-R
Yang P, Luo JB, Wang ZZ, Zhang LL, Feng J, **e XB, Shi QS, Zhang XG (2021) Synthesis, molecular docking, and evaluation of antibacterial activity of 1,2,4-triazole-norfloxacin hybrids. Bioorg Chem 115:105270. https://doi.org/10.1016/j.bioorg.2021.105270
Shi J, Ding M, Luo N, Wan S, Li P, Li J, Bao X (2020) Design, synthesis, crystal structure, and antimicrobial evaluation of 6-fluoroquinazolinylpiperidinyl-containing 1,2,4-triazole Mannich base derivatives against phytopathogenic bacteria and fungi. J Agric Food Chem 68:9613–9623. https://doi.org/10.1021/acs.jafc.0c01365
Amin NH, El-Saasi MT, Ibrahim AA, Abdel-Rahman HM (2021) Design, synthesis and mechanistic study of new 1,2,4-triazole derivatives as antimicrobial agents. Bioorg Chem 111:104841. https://doi.org/10.1016/j.bioorg.2021.104841
Bitla S, Gayatri AA, Puchakayala MR, Bhukya VK, Vannada J, Dhanavath R, Kuthati B, Kothula D, Sagurthi SR, Atcha KR (2021) Design and synthesis, biological evaluation of bis-(1,2,3- and 1,2,4)-triazole derivatives as potential antimicrobial and antifungal agents. Bioorg Med Chem Lett 41:128004. https://doi.org/10.1016/j.bmcl.2021.128004
Fan Z, Shi J, Bao X (2018) Synthesis and antimicrobial evaluation of novel 1,2,4-triazole thioether derivatives bearing a quinazoline moiety. Mol Divers 22:657–667. https://doi.org/10.1007/s11030-018-9821-8
Long ZQ, Yang LL, Zhang JR, Liu ST, **e J, Wang PY, Zhu JJ, Shao WB, Liu LW, Yang S (2021) Fabrication of versatile pyrazole hydrazide derivatives bearing a 1,3,4-oxadiazole core as multipurpose agricultural chemicals against plant fungal, oomycete, and bacterial diseases. J Agric Food Chem 69:8380–8393. https://doi.org/10.1021/acs.jafc.1c02460
Elbarbary AA, Kenawy ER, Hamada EGI, Edries TB, Meshrif WS (2021) Insecticidal activity of some synthesized 1,3,4-oxadiazole derivatives grafted on chitosan and polymethylmethacrylate against the cotton leafworm Spodoptera littoralis. Int J Biol Macromol 180:539–546. https://doi.org/10.1016/j.ijbiomac.2021.03.050
Peng F, Liu T, Wang Q, Liu F, Cao X, Yang J, Liu L, **e C, Xue W (2021) Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives. J Agric Food Chem 69:11085–11094. https://doi.org/10.1021/acs.jafc.1c03755
Meng HW, Shen ZB, Meng XS, Wei L, Yin ZQ, Wang XR, Zou TF, Liu ZG, Wang TX, Zhang S, Chen YL, Yang XX, Li QS, Duan YJ (2023) Novel flavonoid 1,3,4-oxadiazole derivatives ameliorate MPTP-induced Parkinson’s disease via Nrf2/NF-κB signaling pathway. Bioorg Chem 138:106654. https://doi.org/10.1016/j.bioorg.2023.106654
Nayak S, Gaonkar SL, Hazra D, Chawla K, Hari G, Pai KSR, Guru BR, Hakimane SS (2022) Synthesis, molecular docking and evaluation of 1,3,4-oxadiazole-isobenzofuran hybrids as antimicrobial and anticancer agents. Chem Biodivers 19:e202100956. https://doi.org/10.1002/cbdv.202100956
Dong J, Gao W, Li K, Hong Z, Tang L, Han L, Wang Z, Fan Z (2022) Design, synthesis, and biological evaluation of novel Psoralen-based 1,3,4-oxadiazoles as potent fungicide candidates targeting pyruvate kinase. J Agric Food Chem 70:3435–3446. https://doi.org/10.1021/acs.jafc.1c07911
Khanfar MA (2022) Oxadiazol-based mTOR inhibitors with potent antiproliferative activities: synthetic and computational modeling. Mol Divers 26:3357–3364. https://doi.org/10.1007/s11030-021-10367-4
Wang S, Chen J, Shi J, Wang Z, Hu D, Song B (2021) Novel cinnamic acid derivatives containing the 1,3,4-oxadiazole moiety: design, synthesis, antibacterial activities, and mechanisms. J Agric Food Chem 69:11804–11815. https://doi.org/10.1021/acs.jafc.1c03087
Ji J, Shao WB, Chu PL, **ang HM, Qi PY, Zhou X, Wang PY, Yang S (2022) 1,3,4-Oxadiazole derivatives as plant activators for controlling plant viral diseases: preparation and assessment of the effect of auxiliaries. J Agric Food Chem 70:7929–7940. https://doi.org/10.1021/acs.jafc.2c01988
Wang YE, Yang D, Dai L, Huo J, Chen L, Kang Z, Mao J, Zhang J (2022) Design, synthesis, herbicidal activity, and molecular docking study of 2-thioether-5-(thienyl/pyridyl)-1,3,4-oxadiazoles as potent transketolase inhibitors. J Agric Food Chem 70:2510–2519. https://doi.org/10.1021/acs.jafc.1c06897
Pan N, Wu R, Yan C, Zhou M, Fei Q, Li P, Wu W (2023) Design, synthesis, antifungal activity, and molecular docking of novel trifluoromethyl pyrimidine derivatives containing 1,3,4-oxadiazole and thioether moieties as potential succinate dehydrogenase inhibitors. J Heterocycl Chem 60:1768–1777. https://doi.org/10.1002/jhet.4719
Liu C, Fei Q, Pan N, Wu W (2022) Design, synthesis, and antifungal activity of novel 1,2,4-Triazolo[4,3-c]trifluoromethylpyrimidine derivatives bearing the thioether moiety. Front Chem 10:939644. https://doi.org/10.3389/fchem.2022.939644
Wu WN, Jiang YM, Fei Q, Du HT, Yang MF (2020) Synthesis and antifungal activity of novel 1,2,4-triazole derivatives containing an amide moiety. J Heterocycl Chem 57:1379–1386. https://doi.org/10.1002/jhet.3874
Wu WN, Jiang YM, Fei Q, Du HT (2019) Synthesis and fungicidal activity of novel 1,2,4-triazole derivatives containing a pyrimidine moiety. Phosphorus Sulfur Silicon Relat Elem 194:1171–1175. https://doi.org/10.1080/10426507.2019.1633321
Bogolubsky AV, Moroz YS, Mykhailiuk PK, Ostapchuk EN, Rudnichenko AV, Dmytriv YV, Bondar AN, Zaporozhets OA, Pipko SE, Doroschuk RA, Babichenko LN, Konovets AI, Tolmachev A (2015) A one-pot parallel synthesis of alkyl sulfides, sulfoxides, and sulfones. ACS Comb Sci 17:348–354. https://doi.org/10.1021/acscombsci.5b00024
Ding M, Wan S, Wu N, Yan Y, Li J, Bao X (2021) Synthesis, structural characterization, and antibacterial and antifungal activities of novel 1,2,4-triazole thioether and thiazolo[3,2-b]-1,2,4-triazole derivatives bearing the 6-fluoroquinazolinyl moiety. J Agric Food Chem 69:15084–15096. https://doi.org/10.1021/acs.jafc.1c02144
Wei C, Huang J, Luo Y, Wang S, Wu S, **ng Z, Chen J (2021) Novel amide derivatives containing an imidazo[1,2-a]pyridine moiety: design, synthesis as potential nematicidal and antibacterial agents. Pestic BioChem Phys 175:104857. https://doi.org/10.1016/j.pestbp.2021.104857
Liu HW, Ji QT, Ren GG, Wang F, Su F, Wang PY, Zhou X, Wu ZB, Li Z, Yang S (2020) Antibacterial functions and proposed modes of action of novel 1,2,3,4-tetrahydro-β-carboline derivatives that possess an attractive 1,3-diaminopropan-2-ol pattern against rice bacterial blight, kiwifruit bacterial canker, and citrus bacterial canker. J Agric Food Chem 68:12558–12568. https://doi.org/10.1021/acs.jafc.0c02528
Wan S, Wu N, Yan Y, Yang Y, Tian G, An L, Bao X (2023) Design, synthesis, crystal structure, and in vitro antibacterial activities of sulfonamide derivatives bearing the 4-aminoquinazoline moiety. Mol Divers 27:1243–1254. https://doi.org/10.1007/s11030-022-10484-8
Jeschke P (2023) Recent developments in fluorine-containing pesticides. Pest Manag Sci. https://doi.org/10.1002/ps.7921
Wu CC, Wang BL, Liu JB, Wei W, Li YX, Liu Y, Chen MG, **ong LX, Yang N, Li ZM (2017) Design, synthesis and insecticidal activities of novel anthranilic diamides containing fluorinated groups as potential ryanodine receptors activitors. Chin Chem Lett 28:1248–1251. https://doi.org/10.1016/j.cclet.2017.01.019
Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA (2015) Applications of fluorine in medicinal chemistry. J Med Chem 58:8315–8359. https://doi.org/10.1021/acs.jmedchem.5b00258
Hilario E, Keyser SD, Fan L (2020) Structural and biochemical characterization of a glutathione transferase from the citrus canker pathogen Xanthomonas. Acta Cryst D 76:778–789. https://doi.org/10.1107/S2059798320009274
Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5:193–198. https://doi.org/10.1016/S1360-1385(00)01601-0
Acknowledgements
This work was financially supported by Guizhou Provincial Science and Technology Projects (QKH PTRC-CXTD[2022] 002), Education Department of Guizhou Province-Natural Science Research Project (QJJ[2023]042), and Science and Technology Fund Project of Guizhou (QKHJC[2020]1Z023), Guizhou Provincial Science and Technology Projects (QKHCG[2023]YB090), and Guiyang University Graduate Student Innovation Project (GYU-YJS[2021]-38).
Author information
Authors and Affiliations
Contributions
QF, CL, and YL: They proceeded the organic synthesis, bioassay, and data analysis. HC: He provided help for the design of bioassay and data analysis and wrote the manuscript. FM: He had contributed to characterization of compounds. SX and WW: They proposed the idea, analyzed the data, provided funding support, and revised the manuscript. All authors agreed with the publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fei, Q., Liu, C., Luo, Y. et al. Rational design, synthesis, and antimicrobial evaluation of novel 1,2,4-trizaole-substituted 1,3,4-oxadiazole derivatives with a dual thioether moiety. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10848-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10848-2