Abstract
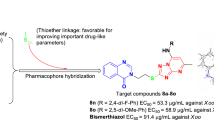
A series of novel quinazolin-4-one derivatives (7a–7n) bearing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety were designed, synthesized and evaluated for their inhibition activities against phytopathogenic bacteria and fungi in vitro. All of the target compounds were fully characterized through \(^{1}\hbox {H}\) NMR, \(^{13}\hbox {C}\) NMR, HRMS and IR spectra. Among these compounds, the structure of compound 7e was unambiguously confirmed via single-crystal X-ray diffraction analysis. The turbidimetric assays indicated that compounds 7b, 7d, 7g, 7k and 7n exhibited much more potent inhibition activities against the pathogen Xanthomonas oryzae pv. oryzae (Xoo), relative to control Bismerthiazol. Moreover, antibacterial activities of compounds 7j, 7k and 7n against the pathogen Xanthomonas axonopodis pv. citri (Xac) were comparable to that of control Bismerthiazol. As for the pathogen Ralstonia solanacearum (Rs), only compounds 7g and 7i demonstrated inhibition activities similar to control Thiadiazole-copper. Moreover, this class of compounds did not display inhibition activity against three fungi tested. The above findings indicated that quinazolin-4-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety have a potential as promising candidates for the development of new and more efficient agricultural bactericides.
Graphical Abstract





Similar content being viewed by others
References
Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S, Zhang Q (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37:517–527. doi:10.1046/j.1365-313X.2003.01976.x
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5:1–15. doi:10.1046/j.1364-3703.2003.00197.X
Ryan RP, Vorhölter FJ, Potnis N, Jones JB, Van Sluys MA, Bogdanove AJ, Dow JM (2011) Pathogenomics of Xanthomonas: understanding bacterium—plant interactions. Nat Rev Microbiol 9:344–355. doi:10.1038/nrmicro2558
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320. doi:10.1007/s001220050565
Li J, Wang N (2011) Genome-wide mutagenesis of Xanthomonas axonopodis pv. citri reveals novel genetic determinants and regulation mechanisms of biofilm formation. PLos One 6:e21804. doi:10.1371/journal.pone.0021804
Tiwary BK, Pradhan K, Nanda AK, Chakraborty R (2015) Implication of quinazoline-\(4(3H)\)-ones in medicinal chemistry: a brief review. J Chem Biol Ther 1:104–110. doi:10.4172/2572-0406.1000104
Wang X, Li P, Li Z, Yin J, He M, Xue W, Chen Z, Song B (2013) Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(\(p\)-trifluoromethoxy)anilino]-\(4(3H)\)-quinazolinone moiety. J Agric Food Chem 61:9575–9582. doi:10.1021/jf403193q
Bouley R, Ding D, Peng Z, Bastian M, Lastochkin E, Song W, Suckow MA, Schroeder VA, Wolter WR, Mobashery S, Chang M (2016) Structure-activity relationship for the \(4(3H)\)-quinazolinone antibacterials. J Med Chem 59:5011–5021. doi:10.1021/acs.jmedchem.6b00372
Zhang J, Liu J, Ma Y, Ren D, Cheng P, Zhao J, Zhang F, Yao Y (2016) One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg Med Chem Lett 26:2273–2277. doi:10.1016/j.bmcl.2016.03.052
Chen M, Li P, Hu D, Zeng S, Li T, ** L, Xue W, Song B (2016) Synthesis, antiviral activity, 3D-QSAR, and interaction mechanisms study of novel malonate derivatives containing quinazolin-\(4(3H)\)-one moiety. Bioorg Med Chem Lett 26:168–173. doi:10.1016/j.bmcl.2015.11.006
Deev SL, Yasko MV, Karpenko IL, Korovina AN, Khandazhinskaya AL, Andronova VL, Galegov GA, Shestakova TS, Ulomskii EN, Rusinov VL, Chupakhin ON, Kukhanova MK (2010) 1,2,4-Triazoloazine derivatives as a new type of herpes simplex virus inhibitors. Bioorg Chem 38:265–270. doi:10.1016/j.bioorg.2010.09.002
Ramírez-Macías I, Marín C, Salas JM, Caballero A, Rosales MJ, Villegas N, Rodríguez-Dieguez A, Barea E, Sánchez-Moreno M (2011) Biological activity of three novel complexes with the ligand 5-methyl-1,2,4-triazolo[1,5-\(a\)]pyrimidin- \(7(4H)\)-one against leishmania spp. J Antimicrob Chemother 66:813–819. doi:10.1093/jac/dkq537
Caballero AB, Marín C, Rodríguez-Diéguez A, Ramírez-Macías I, Barea E, Sánchez-Moreno M, Salas JM (2011) In vitro and in vivo antiparasital activity against Trypanosoma cruzi of three novel 5-methyl-1,2,4-triazolo[1,5-\(a\)]pyrimidin-\(7(4H)\)-one-based complexes. J Inorg Biochem 105:770–776. doi:10.1016/j.**orgbio.2011.03.015
Bedingfield PTP, Cowen D, Acklam P, Cunningham F, Parsons MR, Mcconkey GA, Fishwick CWG, Johnson AP (2012) Factors influencing the specificity of inhibitor binding to the human and malaria parasite dihydroorotate dehydrogenases. J Med Chem 55:5841–5850. doi:10.1021/jm300157n
Ruiz J, Villa MD, Cutillas N, López G, de Haro C, Bautista D, Moreno V, Valencla L (2008) Palladium(II) and platinum(II) organometallic complexes with 4,7-dihydro-5-methyl-7-oxo[1,2,4]triazolo[1,5-\(a\)]pyrimidine. Antitumor activity of the platinum compounds. Inorg Chem 47:4490–4505. doi:10.1021/ic701873b
Yan BR, Lv XY, Du H, Gao MN, Huang J, Bao XP (2016) Synthesis and biological activities of novel quinazolinone derivatives containing a 1,2,4-triazolylthioether moiety. Chem Pap 70:983–993. doi:10.1515/chempap-2016-0034
Pan D, Du H, Lü X, Bao X (2016) Synthesis and antibacterial activities of novel quinazoline-2,4-dione derivatives containing the 1,2,4-triazole Schiff-base unit. Chin J Org Chem 36:818–825. doi:10.6023/cjoc201510005
Yan B, Lü X, Du H, Bao X (2016) Design, synthesis and biological activities of novel quinazolinone derivative bearing 4-phenyl-5-thioxo-1,2,4-triazole Mannich bases. Chin J Org Chem 36:207–212. doi:10.6023/cjoc201506026
Liu J, Liu Y, Jian J, Bao X (2013) Synthesis and fungicidal activities of novel quinazoline derivatives containing 1,2,4-triazole Schiff-base unit. Chin J Org Chem 33:370–374. doi:10.6023/cjoc201209023
Xu H, Wang YY (2010) Antifungal agents. Part 5: Synthesis and antifungal activities of aminoguanidine derivatives of \(N\)-arylsulfonyl-3-acylindoles. Bioorg Med Chem Lett 20:7274–7277. doi:10.1016/j.bmcl.2010.10.084
Xu H, Fan LL (2011) Antifungal agents. Part 4: Synthesis and antifungal activities of novel indole[1,2-\(c\)]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro. Eur J Med Chem 46:364–369. doi:10.1016/j.ejmech.2010.10.022
Yao YP, Dai FY, Dong KK, Mao Q, Wang YL, Chen T (2011) Synthesis and antibacterial activities of pleuromutilin derivatives with quinazolinone and thioether groups. J Chem Res 35:4–7. doi:10.3184/174751911X556675
Xu WM, Han FF, He M, Hu DY, He J, Yang S, Song BA (2012) Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J Agric Food Chem 60:1036–1041. doi:10.1021/jf203772d
Li P, Shi L, Yang X, Yang L, Chen XW, Wu F, Shi QC, Xu WM, He M, Hu DY, Song BA (2014) Design, synthesis, and antibacterial activity against rice bacterial leaf blight and leaf streak of 2,5-substituted-1,3,4 -oxadiazole/thiadiazole sulfone derivative. Bioorg Med Chem Lett 24:1677–1680. doi:10.1016/j.bmcl.2014.02.060
Wang X, Yin J, Shi L, Zhang G, Song B (2014) Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-\(4(3H)\)-one. Eur J Med Chem 77:65–74. doi:10.1016/j.ejmech.2014.02.053
Chen CJ, Song BA, Yang S, Xu GF, Bhadury PS, ** LH, Hu DY, Li QZ, Liu F, Xue W, Lu P, Chen Z (2007) Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg Med Chem 15:3981–3989. doi:10.1016/j.bmc.2007.04.014
Fan Z, Shi Z, Zhang H, Liu X, Bao L, Ma L, Zuo X, Zheng Q, Mi N (2009) Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance. J Agric Food Chem 57:4279–4286. doi:10.1021/jf8031364
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21362003) and the Agricultural Research Projects of Guizhou Province (No. 20093010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11030_2017_9782_MOESM1_ESM.doc
Supplementary data The original spectral files (including 1H NMR, 13C NMR and HRMS) of intermediates 5 & 6as well as target compounds 7a-7n can be found in this section. 294 bytes
Rights and permissions
About this article
Cite this article
Du, H., Fan, Z., Yang, L. et al. Synthesis of novel quinazolin-4(3H)-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety as effective agricultural bactericides against the pathogen Xanthomonas oryzae pv. oryzae . Mol Divers 22, 1–10 (2018). https://doi.org/10.1007/s11030-017-9782-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9782-3