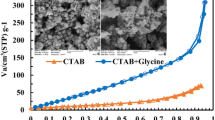
Abstract
Cetyltrimethylammonium bromide (CTAB) was used as fuel in solution combustion synthesis of LiFePO4 powders at various fuel contents (ϕ = 1, 2, and 3). Both conventional and microwave heating methods were applied for ignition of the precursor solution. The slow combustion reaction rate characterized by thermal analysis guaranteed the direct formation of the LiFePO4 phase. Phase evolution studies by X-ray powder diffraction showed that nearly single-phase LiFePO4 powders were obtained at higher fuel contents. The as-combusted powders were calcined at 700 °C for removing the impurity phases. The specific surface area of conventionally combusted powders (137 m2/g) was slightly higher than that for microwave heating, as measured from nitrogen adsorption–desorption isotherms. The higher discharge capacity (91 mAh/g) and higher rate capability were obtained by the microwave-combusted LiFePO4 powders due to their higher crystallinity and lower electrode resistance, as confirmed by electrochemical impedance spectroscopy.

Initial discharge curves of the as-calcined LiFePO4 powders at 0.1 C rate.
Highlights
-
LiFePO4 powders were prepared by solution combustion synthesis method.
-
Conventional and microwave ignition were compared.
-
Higher crystallinity of the microwave-combusted powders led to the higher discharge capacity.









Similar content being viewed by others
References
Myung S-T, Amine K, Sun Y-K (2015) Nanostructured cathode materials for rechargeable lithium batteries. J Power Sources 283:219–236
Bhaskar A, Mikhailova D, Kiziltas-Yavuz N, Nikolowski K, Oswald S, Bramnik NN, Ehrenberg H (2014) 3d-Transition metal doped spinels as high-voltage cathode materials for rechargeable lithium-ion batteries. Prog Solid State Chem 42:128–148
Zhang W-J (2011) Structure and performance of LiFePO4 cathode materials: A review. J Power Sources 196:2962–2970
TVSL Satyavani, Srinivas Kumar A, PSV SubbaRao (2016) Methods of synthesis and performance improvement of lithium iron phosphate for high rate Li-ion batteries: a review. J Eng Sci Tech 19:178–188
Zhi X, Liang G, Wang L, Ou X, Zhang J, Cui J (2009) The cycling performance of LiFePO4/C cathode materials. J Power Sources 189:779–782
Zhu J, Yoo K, El-halees I, Kisailus D (2014) Solution deposition of thin carbon coatings on LiFePO4. ACS Appl Mater Interfaces 6:21550–21557
Yi T-F, Li X-Y, Liu H, Shu J, Zhu Y-R, Zhu R-S (2012) Recent developments in the do** and surface modification of LiFePO4 as cathode material for power lithium ion battery. Ionics 18:529–539
Li Q, Zheng F, Huang Y, Zhang X, Wu Q, Fu D, Zhang J, Yin J, Wang H (2015) Surfactants assisted synthesis of nano-LiFePO4/C composite as cathode materials for lithium-ion batteries. J Mater Chem A 3:2025–2035
Li X, Shao Z, Liu K, Zhao Q, Liu G, Xu B (2017) Influence of synthesis method on the performance of the LiFePO4/C cathode material. Colloids Surf A Physicochem Eng Asp 529:850–855
Kulka A, Walczak K, Zając W, Molenda J (2017) Effect of reducing agents on low-temperature synthesis of nanostructured LiFePO4. J Solid State Chem 253:367–374
Gao C, Zhou J, Liu G, Wang L (2017) Microwave-assisted synthesis and surface decoration of LiFePO4 hexagonal nanoplates for lithium-ion batteries with excellent electrochemical performance. J Mater Sci 52:1590–1602
Varma A, Mukasyan AS, Rogachev AS, Manukyan KV (2016) Solution combustion synthesis of nanoscale materials. Chem Rev 116:14493–14586
Li F-t, Ran J, Jaroniec M, Qiao SZ (2015) Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale 7:17590–17610
Wen W, Wu J-M (2014) Nanomaterials via solution combustion synthesis: a step nearer to controllability. RSC Adv 4:58090–58100
Pourgolmohammad B, Masoudpanah SM, Aboutalebi MR (2017) Effects of the fuel type and fuel content on the specific surface area and magnetic properties of solution combusted CoFe2O4 nanoparticles. Ceram Int 43:8262–8268
Fathi H, Masoudpanah SM, Alamolhoda S, Parnianfar H (2017) Effect of fuel type on the microstructure and magnetic properties of solution combusted Fe3O4 powders. Ceram Int 43:7448–7453
Pourgolmohammad B, Masoudpanah SM, Aboutalebi MR (2017) Effect of starting solution acidity on the characteristics of CoFe2O4 powders prepared by solution combustion synthesis. J Magn Magn Mater 424:352–358
Parnianfar H, Masoudpanah SM, Alamolhoda S, Fathi H (2017) Mixture of fuels for solution combustion synthesis of porous Fe3O4 powders. J Magn Magn Mater 432:24–29
Zhao B, Yu X, Cai R, Ran R, Wang H, Shao Z (2012) Solution combustion synthesis of high-rate performance carbon-coated lithium iron phosphate from inexpensive iron (iii) raw material. J Mater Chem 22:2900–2907
Radpour M, Alamolhoda S, Masoudpanah SM (2017) Effects of pH value on the microstructure and magnetic properties of solution combusted Fe3O4 powders. Ceram Int 43:13729–13734
Javadi S, Masoudpanah SM, Zakeri A (2016) Conventional versus microwave combustion synthesis of CoFe2O4 nanoparticles. J Sol-Gel Sci Technol 79:176–183
Zhao J, Yan W (2011) Chapter 8 - Microwave-assisted inorganic syntheses, modern inorganic synthetic chemistry, Elsevier, Amsterdam, pp. 173–195
Park KS, Son JT, Chung HT, Kim SJ, Lee CH, Kim HG (2003) Synthesis of LiFePO4 by co-precipitation and microwave heating. Electrochem Commun 5:839–842
Jain SR, Adiga KC, Pai Verneker VR (1981) A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust Flame 40:71–79
Vasei HV, Masoudpanah SM, Adeli M, Aboutalebi MR (2018) Solution combustion synthesis of ZnO powders using CTAB as fuel. Ceram Int 44:7741–7745
Famenin Nezhad Hamedani S, Masoudpanah SM, Bafghi MS, Asgharinezhad Baloochi N (2018) Solution combustion synthesis of CoFe2O4 powders using mixture of CTAB and glycine fuels. J Sol-Gel Sci Technol 86:743–750
Hadadian S, Masoudpanah SM, Alamolhoda S (2018) Solution combustion synthesis of Fe3O4 powders using mixture of CTAB and citric acid fuels, J Superconductivity Novel Magnetism 32:353–360
Yin W, Wang W, Zhou L, Sun S, Zhang L (2010) CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. J Hazard Mater 173:194–199
Nersisyan HH, Lee JH, Ding J-R, Kim K-S, Manukyan KV, Mukasyan AS (2017) Combustion synthesis of zero-, one-, two- and three-dimensional nanostructures: current trends and future perspectives. Prog Energy Combust Sci 63:79–118
Yu F, Zhang L, Zhu M, An Y, **a L, Wang X, Dai B (2014) Overwhelming microwave irradiation assisted synthesis of olivine-structured LiMPO4 (M=Fe, Mn, Co and Ni) for Li-ion batteries. Nano Energy 3:64–79
Vranken T, Van Gompel W, D’Haen J, Van Bael MK, Hardy A (2017) Aqueous solution–gel precursors for LiFePO lithium ion battery cathodes, their decomposition and phase formation. J Sol-Gel Sci Technol 84:198–205
Julien C, Mauger A, Vijh A, Zaghib K (2016) Lithium Batteries, Springer International Publishing, Switzerland
Ait Salah A, Jozwiak P, Zaghib K, Garbarczyk J, Gendron F, Mauger A, Julien CM (2006) FTIR features of lithium-iron phosphates as electrode materials for rechargeable lithium batteries. Spectrochim Acta A 65:1007–1013
Burba CM, Frech R (2004) Raman and FTIR Spectroscopic Study of Li x FePO4 (0 ⩽ x ⩽ 1). J Electrochem Soc 151:A1032–A1038
Makhlouf MT, Abu-Zied BM, Mansoure TH (2014) Effect of fuel/oxidizer ratio and the calcination temperature on the preparation of microporous-nanostructured tricobalt tetraoxide. Adv Powder Technol 25:560–566
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (2008) Reporting Physisorption Data for Gas/Solid Systems, Handbook of Heterogeneous Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 1217–1230
Pourgolmohammad B, Masoudpanah SM, Aboutalebi MR (2017) Synthesis of CoFe2O4 powders with high surface area by solution combustion method: effect of fuel content and cobalt precursor. Ceram Int 43:3797–3803
Yamada A, Koizumi H, Nishimura S-i, Sonoyama N, Kanno R, Yonemura M, Nakamura T, Kobayashi Y (2006) Room-temperature miscibility gap in LixFePO4. Nat Mater 5:357
Calderón CA, Thomas JE, Lener G, Barraco DE, Visintin A (2017) Electrochemical comparison of LiFePO4 synthesized by a solid-state method using either microwave heating or a tube furnace. J Appl Electrochem 47:1179–1188
** Y-C, Duh J-G (2013) Nanostructured LiNi0.5Mn1.5O4 cathode material synthesized by polymer-assisted co-precipitation method with improved rate capability. Mater Lett 93:77–80
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghi, Z., Masoudpanah, S.M. CTAB-assisted solution combustion synthesis of LiFePO4 powders. J Sol-Gel Sci Technol 91, 335–341 (2019). https://doi.org/10.1007/s10971-019-05002-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05002-6