Abstract
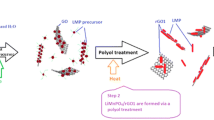
Nanosized hybrid cathode materials, LiMn0.7Fe0.3PO4 with olivine structure anchoring on the graphene matrices, were facilely prepared by employing a modified microwave-assisted solvothermal method. Many measurements such as XRD, SEM, TEM, EDS, Raman spectra and XPS have been utilized to identify their physicochemical properties. Electron microscopy analyses revealed that the widely distributed LiMn0.7Fe0.3PO4 nanorods were selectively and homogeneously grown on graphene sheets with rod length of 100–200 nm and diameter of 30–50 nm. In particular, it was fully improved that the deliberate additions of conductive matrix, graphene oxide, have facilitated the specific growth of LiMn0.7Fe0.3PO4 and therefore improved their homogeneity and morphology to form a huge electric network. Electrochemical assessments indicated that the as-synthesized materials delivered an initial discharge capacity of 159.8 mAh g−1 at 0.1 C and even 81.6 mAh g−1 at 20 C, meanwhile maintained their excellent rate capability and cycling ability, about 91.7% capacity retention after 80 cycles at 1 C. Theoretically speaking, the excellent electrochemical performance maybe makes these nanosized LiMn0.7Fe0.3PO4 cathode materials a potential candidate for the practical implications in high-power devices and energy storage systems.











Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phosphor—olivines as positive—electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Subramanya HP, Ellis B, Coombs N, Nazar LF (2004) Nano-network electronic conduction in iron and nickel olivine phosphates. Nat Mater 3:147–152
Yuan LX, Wang ZH, Zhang WX, Hu XL, Chen JT, Huang YH, Goodenough JB (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ Sci 4:269–284
Song H-K, Lee KT, Kim MG, Nazar LF, Cho J (2010) Recent progress in nanostructured cathode materials for lithium secondary batteries. Adv Funct Mater 20:3818–3834
Chung S-Y, Bloking JT, Ching Y-M (2002) Electronically conductive phospho-olivines as lithium storage electrodes. Nat Mater 1:123–128
Martha SK, Markovsky B, Grinblat J, Gofer Y, Haik O, Zinigrad E, Aurbach D, Drezen T, Wang D, Deghenghi G, Exnar I (2009) LiMnPO4 as an advanced cathode material for rechargeable lithium batteries. J Electrochem Soc 156:A541–A552
Okada S, Sawa S, Egashira M, Yamaki J, Tabuchi M, Kageyama H, Konishi T, Yoshino A (2001) Cathode properties of phospho-olivine LiMPO4 for lithium secondary batteries. J Power Sources 97–98:430–432
Oh SM, Oh SW, Yoon CS, Scrosati B, Amine K, Sun YK (2010) High-performance carbon-LiMnPO4 nanocomposite cathode for lithium batteries. Adv Funct Mater 20:3260–3265
Zou QQ, Zhu GN, **a YY (2012) Preparation of carbon-coated LiFe0.2Mn0.8PO4 cathode material and its application in a novel battery with Li4Ti5O12 anode. J. Power Sources. 206:222–229
Sun Y-K, Oh S-M, Park H-K, Scrosati B (2011) Micrometer-sized, nanoporous, high-volumetric-capacity LiMn0.85Fe0.15PO4 cathode material for rechargeable lithium-ion batteries. Adv Mater 23:5050–5054
Oh S-M, Myung S-T, Park JB, Scrosati B, Amine K, Sun Y-K (2012) Double-structured LiMn0.85Fe0.15PO4 coordinated with LiFePO4 for rechargeable lithium batteries. Angew Chem Int Ed 51:1853–1856
Wang H, Yang Y, Liang Y, Cui L-F, Casalongue HS, Li Y, Hong G, Cui Y, Dai H (2011) LiMn1−x Fe x PO4 Nanorods grown on graphene sheets for ultrahigh-rate-performance lithium ion batteries. Angew Chem Int Ed 50:7364–7368
Jensen KMO, Christensen M, Gunnlaugsson HP, Lock N, Bojesen ED, Proffen T, Iversen BB (2013) Defects in hydrothermally synthesized LiFePO4 and LiFe1−x Mn x PO4 cathode materials. Chem Mater 25:2282–2290
Ding B, **ao P, Ji G, Ma Y, Lu L, Lee JY (2013) High-Performance lithium-ion cathode LiMn0.7Fe0.3PO4/C and the mechanism of performance enhancements through Fe substitution. ACS Appl Mater Interfaces 5:12120–12126
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Wang H, Robinson JT, Diankov G, Dai H (2010) Nanocrystal growth on graphene with various degrees of oxidation. J Am Chem Soc 132:3270–3271
Liang Y, Wang H, Casalongue HS, Chen Z, Dai H (2010) TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 3:701–705
Cundy CS, Cox PA (2003) The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem Rev 103:663–702
Zhang B, Wang X, Liu Z, Li H, Huang X (2010) Enhanced electrochemical performances of carbon coated mesoporous LiFe0.2Mn0.8PO4. J Electrochem Soc 157:A285–A288
Tang KB, Qian YT, Zeng JH, Yang XG (2003) Solvothermal route to semiconductor nanowires. Adv Mater 15:448–450
Zhu Y-J, Chen F (2014) Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem Rev 114:6462–6555
Nan CY, Lu J, Chen C, Peng Q, Li YD (2011) Solvothermal synthesis of lithium iron phosphate nanoplates. J Mater Chem 21:9994–9996
Ellis B, Kan KH, Makahnouk WRM, Nazar LF (2007) Synthesis of nanocrystals and morphology control of hydrothermally prepared LiFePO4. J Mater Chem 17:3248–3254
Wilcox JD, Doeff MM, Marcinek M, Kostecki R (2007) Factors influencing the quality of carbon coatings on LiFePO4. J Electrochem Soc 154:A389–A395
Yang M, Guo Y, Wang Q, **e J (2014) Synthesis and properties of optimized LiFePO4/C by a CVD-assisted two-step coating method. J Nanopart Res 16:1–9
Yan Y, Yin YX, **n S, Guo YG, Wan LJ (2012) Ionothermal synthesis of sulfur-doped porous carbons hybridized withgraphene as superior anode materials for lithium-ion batteries. Chem Commun 48:10663–10665
Yun YS, Le VD, Kim H, Chang SJ, Baek SJ, Park S, Kim BH, Kim YH, Kang K, ** HJ (2014) Effects of sulfur do** on graphene-based nanosheets for use as anode materials in lithium-ion batteries. J Power Sources 262:79–85
Guo S-M, Liu J-R, Qiu S, Wang Y-R, Yan X-R, Wu N-N, Wang S-Y, Guo Z-H (2016) Enhancing electrochemical performances of TiO2 porous microspheres through hybridizing with FeTiO3 and nanocarbon. Electrochim Acta 190:556–565
Wang Y-R, He Q-L, Guo J, Wang J-M, Luo Z-P, Shen TD, Ding K-Q, Khasanov A, Wei S-Y, Guo Z-H (2015) Ultrafine FePd nanoalloys decorated multiwalled cabon nanotubes toward enhanced ethanol oxidation reaction. ACS Appl Mater Interfaces 7:23920–23931
Bhuvaneswari MS, Bramnik NN, Ensling D, Ehrenberg H, Jaegermann W (2008) Synthesis and characterization of carbon nano fiber/LiFePO4 composites for Li-ion batteries. J Power Sources 180:553–560
Castro L, Dedryvere R, Elkhalifi M, Lippens P, Breger J, Tessier C, Gonbeau D (2010) The spin-polarized electronic structure of LiFePO4 and FePO4 evidenced by in-lab XPS. J Phys Chem C 114:17995–18000
Chang X, Wang Z, Li X, Zhang L, Guo H, Peng W (2005) Synthesis and performance of LiMn0.7Fe0.3PO4 cathode material for lithium ion batteries. Mater Res Bull 40:1513–1520
Zaghib K, Mauger A, Gendron F, Massot M, Julien CM (2008) Insertion properties of LiFe0.5Mn0.5PO4 electrode materials for Li-ion batteries. Ionics 14:371–376
Martha SK, Grinblat J, Haik O, Zinigrad E, Drezen T, Miners JH, Exnar I, Kay A, Markovsky B, Aurbach D (2009) LiMn0.8Fe0.2PO4, an advanced cathode material for rechargeable lithium batteries. Angew Chem Int Ed 121:8711–8715
Wang Y, Zhang D, Yu X, Cai R, Shao Z, Liao X, Ma Z (2010) Mechanoactivation-assisted synthesis and electrochemical characterization of manganese lightly doped LiFePO4. J. Alloys. Compd. 492:675–680
Jang BZ, Liu CG, Neff D, Ming ZN, Wang C, **ong W, Zhamu A (2011) Graphene surface-enabled lithium ion-exchanging cells: next-generation high-power energy storage devices. Nano Lett 11:3785–3791
Zhou X, **e Y, Deng Y, Qin X, Chen G (2015) The enhanced rate performance of LiFe0.5Mn0.5PO4/C cathode material via synergistic strategies of surfactant-assisted solid state method and carbon coating. J Mater Chem A 3:996–1004
Liu T, Xu JJ, Wu BB, **a QB, Wu XD (2013) Porous LiMn0.7Fe0.3PO4/C prepared by a thermal decomposition method as high performance cathode materials for Li-ion batteries. RSC Adv 3:13337–13341
Wang X, Cao XQ, Bourgeois L, Guan H, Chen SM, Zhong YT, Tang DM, Li HQ, Zhai TY, Li L, Bando Y, Golberg D (2012) N-doped graphene-SnO2 sandwich paper for high-performance lithium-ion batteries. Adv Funct Mater 22:2682–2690
Xu DW, Chu XD, He YB, Ding ZJ, Li BH, Han WJ, Du HD, Kang FY (2015) Enhanced performance of interconnected LiFePO4/C microspheres with excellent multiple conductive network and subtle mesoporous structure. Electrochim Acta 152:398–407
Yang X, Mi Y, Zhang W, Wu B, Zhou H (2015) Enhanced electrochemical performance of LiFe0.6Mn0.4PO4/C cathode material prepared by ferrocene-assisted calcination process. J Power Sources 275:823–830
Gibot P, Casas-Cabanas M, Laffont L, Levasseur S, Carlach P, Hamelet S, Tarascon J-M, Masquelier C (2008) Room-temperature single-phase Li insertion/extraction in nanoscale Li x FePO4. Nat Mater 7:741–747
Wu G, Zhou Y, Gao X, Shao Z (2013) Facile low-temperature polyol process for LiFePO4 nanoplate and carbon nanotube composite. Solid State Sci 2:15–20
Acknowledgement
This work was financially supported by the Natural Science Foundation of Chongqing (Grants No. 2014jcyjA50036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, B., Gao, W. LiMn0.7Fe0.3PO4 nanorods grown on graphene sheets synthesized in situ by modified microwave-assisted solvothermal method as high-performance cathode materials. J Mater Sci 53, 4433–4443 (2018). https://doi.org/10.1007/s10853-017-1835-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1835-6