Abstract
The presence of pharmaceuticals in ecosystems is a major health issue, calling for advanced methods to clean wastewater before effluents reach rivers. Here, we review advanced adsorption methods to remove ibuprofen, with a focus on ibuprofen occurrence and toxicity, adsorbents, kinetics, and adsorption isotherms. Adsorbents include carbon- and silica-based materials, metal–organic frameworks, clays, polymers, and bioadsorbents. Carbon-based adsorbents allow the highest adsorption of ibuprofen, from 10.8 to 408 mg/g for activated carbon and 2.5–1033 mg/g for biochar. Metal–organic frameworks appear promising due to their high surface areas and tunable properties and morphology. 95% of published reports reveal that adsorption kinetics follow the pseudo-second-order model, indicating that the adsorption is predominantly governed by chemical adsorption. 70% of published reports disclose that the Langmuir model describes the adsorption isotherm, suggesting that adsorption involves monolayer adsorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the rapid growth of industrialization and human activities has led to pharmaceutical and personal care products emerging as significant pollutants in aquatic environments, posing a serious global concern. These compounds, along with their metabolites, enter water bodies through various sources such as households, hospitals, factories, and sewage treatment plants, resulting in detrimental effects on water quality and causing substantial harm to aquatic ecosystems (Davarnejad et al. 2018; Sophia and Lima 2018). Antibiotics and anti-inflammatory agents, among the various pharmaceutical contaminants, are particularly noteworthy due to their increasing global consumption (Akhil et al. 2021; Anand et al. 2022; Muñiz-González 2021; Nguyen et al. 2022b; Rameshwar et al. 2023).
Ibuprofen, scientifically known as 2-[4-(2-Methylpropyl) phenyl] propanoic acid, holds the distinction of being the third most commonly used drug worldwide, with an annual consumption of approximately 200 tons (Chopra and Kumar 2020). This non-steroidal anti-inflammatory drug is highly sought after in the market and is primarily produced by Shasun Chemicals and Drugs Ltd., with the lot number IBU0307598 (Mestre et al. 2007). It is commonly prescribed for rheumatoid arthritis, osteoarthritis, pain relief, inflammation, and fever management (Shaheen et al. 2022). Ibuprofen has been detected in wastewater and rivers across multiple countries (Brun. et al. 2006; Mestre et al. 2007; Nakada et al. 2006; Vieno et al. 2005), with increasing concentrations observed in wastewater treatment plants and water bodies. Given its bioactive nature, it poses a potential environmental hazard, as shown in Fig. 1.
Fate of ibuprofen in the environment. The daily usage of ibuprofen is steadily rising, leading to a corresponding increase in waste containing ibuprofen. This waste is generated by pharmaceutical industries, hospitals, and households and is often disposed of in sewage systems, landfills, or municipal treatment plants. Unfortunately, this disposal method allows ibuprofen to enter water bodies, where living organisms can absorb it and subsequently enter the food chain. Improper disposal of ibuprofen poses a significant risk of bioaccumulation and ecological harm
Various methods have been explored for the removal of pharmaceutical compounds from different matrices (Caban and Stepnowski 2021; Taoufik et al. 2020), including filtration (Femina Carolin et al. 2021; Gu et al. 2018; Taheran et al. 2016), advanced oxidation processes (Akbari Beni et al. 2020; Bastami et al. 2017; Brillas 2022; Kanakaraju et al. 2014; Sruthi et al. 2021), ion exchange (Jiang et al. 2015), biological treatment (Tiwari et al. 2017), and adsorption (Bello and Raman 2019; Duarte et al. 2022; Osman et al. 2023a; Ranjbari et al. 2020). However, among these methods, adsorption has gained significant attention due to its cost-effectiveness, simplicity, high efficiency, regenerability, and scalability (Ayati et al. 2019; Karimi-Maleh et al. 2021b; Shahinpour et al. 2022). Adsorption has emerged as a superior approach for pharmaceutical compound removal from aqueous solutions (Ahmed 2017; Huang et al. 2021; Igwegbe et al. 2021; Prasetya et al. 2023).
Extensive research has been conducted on removing ibuprofen from aquatic media through adsorption, leading to the exploration of a wide range of adsorbents with diverse origins. These adsorbents include activated carbons (Labuto et al. 2022; Matějová et al. 2022), polymers (Karimi-Maleh et al. 2021a; Yu et al. 2022; Van Tran et al. 2019b). Pyrolysis has enhanced their surface areas and porosities and broadened their applications (Yu et al. 2021). Among them, porous activated carbon derived from the pyrolysis of metal–organic framework-6 and activated with potassium hydroxide has demonstrated a high sorption capacity for pharmaceutical compounds, including ibuprofen (An et al. 2018). Moreover, highly porous nitrogen-/oxygen-doped porous carbons obtained from the zeolitic-imidazolate framework-8, namely ZIF-8, have shown effective performance in the adsorptive removal of antibiotics, such as ciprofloxacin (Li et al. 2017), diclofenac (Bhadra et al. 2017), and ibuprofen (Bhadra et al. 2017). Bhadra et al. (2017) found that H-bonding interactions, mainly through the phenolic group, are responsible for ibuprofen adsorption using zeolitic imidazolate framework-8-derived carbon, with carbon acting as the hydrogen-bond donor and ibuprofen as the H-bond acceptor.
The adsorption capacity of activated carbon was also improved by other parameters, including activation time (Ulfa et al. 2020b) and activator concentration (Ulfa et al. 2020a). One study demonstrated that increasing the activator concentration improved adsorption capacity by precipitating impurities, increasing the surface area, and increasing the functional groups (Ulfa et al. 2020a). Other advanced treatments, such as ozonation (Guillossou et al. 2019) and sonication, have been found to effectively enhance the ibuprofen adsorption capacity of activated carbons (Fröhlich et al. 2018a; Ondarts et al. 2018). These treatments form new binding sites on the activated carbon surface (Yazidi et al. 2019). For example, Fröhlich et al. (2018b) demonstrated that ultrasound-modified activated carbon exhibited a 25% higher adsorption capacity than unmodified activated carbon.
In the adsorption of ibuprofen onto activated carbon, hydrophobic and π-π interactions between the carbon surface and micro-pollutants play a significant role in the adsorption mechanism. While hydrophobic interactions may not be directly responsible for the adsorption of ibuprofen onto activated carbon, their influence cannot be ignored (Kaur et al. 2018; Zhao et al. 2016). In acidic media, dispersive π–π and donor–acceptor interactions occur between the carbonyl groups in activated carbon and the aromatic ring of ibuprofen (Fröhlich et al. 2018b). As expected, ibuprofen adsorption is higher in monopollutant systems than in wastewater effluent due to particle pore blocking and competition for adsorption sites (Guillossou et al. 2020). While the small size of ibuprofen molecules allows for its fast and high sorption, its molecular configuration and adsorbent size will affect adsorption efficiency (Turk Sekulic et al. 2019).
The surface functionalization or crosslinking of activated carbon presented a promising solution for removing pharmaceuticals (Ali et al. 2019; Tian et al. 2022). These modifications can affect both the adsorption kinetics and adsorption capacity. Recent studies have demonstrated the effectiveness of ethylamine- and ethylenediamine-functionalized activated carbon, which possesses basic and hydrophobic surfaces, respectively, in the Langmuir monolayer adsorption of ibuprofen through endothermic and spontaneous processes (Ali et al. 2019). Following the pseudo-second-order model, the equilibrium adsorptions were faster on the functionalized activated carbons than on unmodified activated carbon. However, the maximum sorption capacity was observed to be in the order of activated carbon more than ethylenediamine-functionalized activated carbon more than ethylamine-functionalized activated carbon (An et al. 2018; Fröhlich et al. 2019). In one study, a magnetic nickel ferrite (NiFe2O4)/activated carbon composite with a high surface area of 564 m2/g showed great potential for ibuprofen removal with a maximum adsorption capacity of 261 mg/g (Fröhlich et al. 2019). In such cases, activated carbon serves not only as a support but also actively participates in ibuprofen uptake through physisorption, attributed to its high surface area, or chemisorption, which is facilitated by the presence of heteroatoms on the surface (Pakade et al. 2019). Wasilewska and Deryło-Marczewska (2022) successfully enhanced the adsorption capacity of activated carbon by immobilizing it in calcium alginate. They achieved maximum sorption capacities of 0.873 mmol/g for diclofenac and 0.381 mmol/g for ibuprofen drugs. The samples with higher activated carbon content exhibited increased hygroscopicity, polarity, and superior adsorption rate and capacity. The higher sorption capacity for diclofenac can be attributed to the disparities in adsorbate solubilities. In contrast, the faster removal rate of ibuprofen is attributed to the variance in the molecular sizes of the drugs.
As presented in Table 1, several studies have shown that maximum ibuprofen adsorption occurs under acidic conditions, particularly between pH 2 and 4. This may be due to the repulsion between the negatively charged activated carbon surface in alkaline media and the negatively charged ibuprofen molecules, which hinders adsorption (Dubey et al. 2010). In acidic solutions, excess hydrogen ions neutralize the negative charges on the adsorbent surface, facilitating the diffusion of ibuprofen molecules. Combining activated carbon adsorption systems and biological treatment or hybrid membrane systems was also proposed to remove ibuprofen (Ferrer-Polonio et al. 2020; Kim et al. 2019; Zhang et al. 2019). For example, granular activated carbon has been efficiently used in the pilot- and full-scale hybrid adsorption columns and membrane systems to remove ibuprofen from aquatic media (Jamil et al. 2020; Zhang et al. 2019). Numerous research studies have been conducted to analyze the thermodynamics of ibuprofen adsorption on activated carbons. As shown in Table 1, the presence of negative ΔG° values confirms the viability and spontaneous nature of ibuprofen sorption onto activated carbons. Moreover, highly negative ΔG° values indicate a significant level of favorability in terms of adsorption (Ahmed 2017).
Apart from activated carbon, other carbon-based materials, such as biochar and hydrochar obtained through the pyrolysis and hydrothermal carbonization of biomass wastes, including agricultural waste, have also been investigated for their potential in ibuprofen adsorption (Delgado-Moreno et al. 2021; Osman et al. 2023c; Patel et al. 2022). Table 2 provides an overview of some specific examples of these adsorbents. Several natural waste sources, such as date palm leaflets (Ali et al. 2019), wood waste (Van Limbergen et al. 2022), Cocos nucifera shell (Chakraborty et al. 2019), date palm fiber wood (Van Limbergen et al. 2022), date seeds (Chakraborty et al. 2020), bamboo waste (Reza et al. 2014), waste coffee residue (Shin et al. 2022), almond shells (Show et al. 2021), Quercus brantii (oak), coffee bean husk (Van Limbergen et al. 2022), sugarcane bagasse (Chakraborty et al. 2018b), tamarind seeds (Show et al. 2022a), Albizialebbeck seeds (Sivarajasekar et al. 2018), and kola nut husk (Bello et al. 2020a), have been used as ibuprofen-adsorbent biochars. The main adsorption mechanism involves a combination of acid/base sorbate equilibria and the interaction of carboxylic acid and phenolic hydroxyl sites with varying pH levels (Essandoh et al. 2015).
To enhance the sorption capacity of biochars in the removal of ibuprofen, various pre- and post-treatment methods have been explored (Shin et al. 2021). These methods include physical modifications like ball milling (Chakraborty et al. 2020; Luo et al. 2020), composite formation (Moreno-Pérez et al. 2021), chemical oxidation (Ali et al. 2019), and acid/base modification (Shin et al. 2020). For example, Shin et al. (2021) demonstrated that the reinforced aromatic structure of sodium hydroxide-activated biochar obtained from spent coffee waste facilitated π–π interaction, significantly improving its adsorption capacity. In another study, Chakraborty et al. (2019) presented a study highlighting the effective performance of activated biochar derived from Cocos nucifera shells, which underwent physical and chemical modifications, in the adsorption of ibuprofen. The modified activated biochar demonstrated maximum sorption capacities of 9.7 mg/g and 12.2 mg/g, respectively. Recently, Moreno-Pérez et al. (2021) explored the adsorption potential of a zinc aluminium alloy (ZnAl)/biochar composite for pharmaceutical compounds. Following the Henry isotherm model, they achieved a remarkable adsorption capacity of 1032 mg/g for ibuprofen. The primary mechanism of transport was identified as surface flux within the particles. Activated carbons also exhibited promise as adsorbents due to their numerous surface functional groups, large surface area, and well-developed pore structures, making them suitable for removing ibuprofen molecules from aqueous environments. Notably, commercial activated carbon (Zhao et al. 2018) and activated carbon derived from primary pulp mill sludge (Coimbra et al. 2019) exhibited remarkable behavior in the adsorptive removal of ibuprofen.
Graphene-based materials, such as pristine graphene and graphene oxide, represent a fascinating category of carbonaceous adsorbents with significant promise for removing ibuprofen. Recent studies have revealed the exceptional characteristics of these materials, including their remarkable hydrophobicity, high adsorption capacity, extensive surface area, low toxicity, and recyclability. The nanostructured porous nature of graphene lends itself well to effective ibuprofen adsorption, making it an ideal choice for this application (Amiri et al. 2019; Khalil et al. 2021, 2020; Lou et al. 2010; Lestari et al. 2018; Tabatabaeian et al. 2020). As a result, certain metal–organic frameworks have demonstrated higher removal capacities than commercial activated carbon (Jun et al. 2019; Lin et al. 2018). In addition to their adsorption capabilities, metal–organic frameworks have attracted attention in drug delivery applications, serving as hosts for controlled release (Chávez et al. 2021). This discussion highlights the significant findings and recent contributions in metal–organic framework-based adsorbents, and some noteworthy examples are presented in Table 4.
The adsorption of ibuprofen onto metal–organic frameworks can be attributed to several potential interaction mechanisms. These mechanisms include the formation of Lewis acid/base complexes between the coordinatively unsaturated sites of the metal ions in metal–organic frameworks and the dissociated ibuprofen molecules. Another mechanism involves hydrogen bonding between the carboxyl group of ibuprofen and oxygen atoms within the structure of the metal–organic framework. Additionally, π–π electron donor–acceptor interactions between the metal–organic frameworks and ibuprofen molecules have been considered (Álvarez-Torrellas et al. 2016; Sun et al. 2019). Furthermore, anion-π interactions between the benzene ring of the metal–organic frameworks and the dissociated carboxyl group of ibuprofen are also possible (Ghasemi et al. 2022; Scheytt et al. 2005; Sun et al. 2019; Wei et al. 2018). Sun et al. (2019) conducted density functional theory calculations to analyze the binding energies and typical structures of ibuprofen adsorbed onto zirconium-based metal–organic framework, namely UiO-66, and amino zirconium-based metal–organic framework, namely UiO-66-NH2, metal–organic frameworks. They comprehensively considered all possible interaction mechanisms involved in pharmaceutical adsorption. They found that the binding energies followed the order of π–π interactions more than hydrogen bonding more than Lewis acid/base more than anion-π interactions. Specifically, hydrogen bonding was identified as the primary pharmaceutical adsorption mechanism, including ibuprofen and oxybenzone, onto the iron-based metal framework, namely MIL-101 (Seo et al. 2016).
Most studies on the adsorption of ibuprofen onto metal–organic frameworks focused on MILs types (Cao et al. 2020; Horcajada et al. 2008; Rajab Asadi et al. 2018). For example, a chemically stable metal–organic framework of iron-based metal–framework, namely MIL-53(Fe), efficiently adsorbed ibuprofen molecules with a removal efficiency above 80% under optimal conditions (Nguyen et al. 2019). In another study, Jun et al. (2019) investigated the effectiveness of aluminium terephthalate, namely MIL-53(Al), as an adsorbent for removing ibuprofen. They observed that the aluminum metal in aluminium terephthalate likely formed coordination bonds with the anionic ibuprofen molecules, contributing to hydrophobic and electrostatic interactions. Additionally, they found that the positive surface charge of aluminium terephthalate decreased gradually as the solution pH increased from 3.5 to 9.5. This reduction in surface charge resulted in enhanced hydrophobic interactions between aluminium terephthalate and ibuprofen molecules.
Furthermore, divalent cations, which act as counter-ions for ibuprofen, have been found to enhance the electrostatic interaction between ibuprofen and metal–organic frameworks by bridging the two entities. On the other hand, divalent anions that coexist with ibuprofen can suppress this electrostatic interaction. The proposed adsorption mechanisms for ibuprofen and carbamazepine are depicted in Fig. 4. Remarkably, the copper-doped iron-based metal–organic framework demonstrated a high adsorption capacity for ibuprofen across a wide pH range, with a maximum sorption capacity of 497 mg/g (** countries. Sci Total Environ 660:57–68. https://doi.org/10.1016/j.scitotenv.2018.12.386 " href="/article/10.1007/s10311-023-01647-6#ref-CR31" id="ref-link-section-d87766045e9916">2019; Martín et al. 2019; Obradović et al. 2022).
In such cases, the adsorbed surfactant's chemical nature strongly affected organoclay materials' adsorption properties (Ghemit et al. 2019). For example, organoclay derivatives of Na+-exchanged montmorillonite, which contained benzyldimethyltetradecylammonium as a cationic surfactant and polyoxyethylene (20)oleyl-ether as a non-ionic surfactant, exhibited a certain versatility in the removal of diverse pharmaceuticals from the effluent of a rural wastewater facility in France (De Oliveira et al. 2020). It was proposed as a filter between the transitions from different settling tanks in wastewater treatment plants to improve the removal efficiency (De Oliveira et al. 2020). Benzyldimethyltetradecylammonium- montmorillonite showed a remarkable affinity for anionic pharmaceutical compounds, while cationic pharmaceutical compounds were better adsorbed onto polyoxyethylene (20)oleyl-ether-montmorillonite and Na+-exchanged montmorillonite, with its dual hydrophobic-hydrophilic nature via compensating Na+ cations and the non-ionic surfactant. The intercalation of surfactants within the interlayer space of organoclays created a hydrophobic environment that adsorbed numerous pharmaceuticals through weak π–π and/or van der Waals interactions. Mainly, electrostatic interactions controlled the adsorption of drugs onto the Na+-exchanged montmorillonite, nonionic polyoxyethylene (20)oleyl-ether-montmorillonite, and cationic benzyldimethyltetradecylammonium- montmorillonite organoclay adsorbents.
The cationic octadecylamine surfactant modification of clays, such as Na+-exchanged mica (Martín et al. 2018) and montmorillonite (Malvar et al. 2020), significantly increased their affinity for ibuprofen molecules. Hydrophobic interactions between the surfactant alkyl chains of modified clay and organic compounds play a major role in adsorption, and the incorporation of ibuprofen occurred on the external surface and in the interlayers (Martín et al. 2018). Martín et al. (2019) compared ibuprofen adsorption to octadecylamine-modified montmorillonite and Na+-exchanged mica. While adsorption was faster onto modified montmorillonite, smaller than 5 min, than modified Na+-exchanged Mica, smaller than 60 min, both adsorption kinetics followed the pseudo-second order, indicating chemisorption. Furthermore, the adsorption isotherm of modified montmorillonite corresponded to the Langmuir model, while that of modified Na+-exchanged mica fitted better to the Freundlich model, indicating a difference in the types of adsorption.
In addition to ibuprofen molecules, the efficient adsorption performance of octadecylamine-modified montmorillonite was also demonstrated in the removal of primary ibuprofen metabolites, including 1-hydroxyibuprofen, 2-hydroxyibuprofen, and carboxyibuprofen, from aqueous solution (Malvar et al. 2020). It was mainly due to electrostatic interaction and partitioning in the adsorption mechanism. The maximum adsorption capacities toward ibuprofen and all its primary metabolites within 20–30 min were 64 mg/g, 20 mg/g, 63 mg/g, and 19 mg/g, respectively (Malvar et al. 2020). It should be noted that the sorption capacities were considerably lower in mixture solutions due to competition for adsorbent active sites.
Hexadecyltrimethylammonium is another surfactant used to modify montmorillonite and zeolite to efficiently absorb ibuprofen and salicylic acid at pH 7 (Choi and Shin 2020). Due to the higher organic carbon content of modified montmorillonite, a higher adsorption capacity compared to that of modified zeolite was observed; furthermore, because of the higher hydrophobicity and molar volume of ibuprofen molecules, it showed higher uptake than salicylic acid. Since the anionic speciation of ibuprofen is more prolific at pH 7, higher than pKa, its adsorption onto modified montmorillonite mainly occurs via two-dimensional surface adsorption onto the pseudo-organic medium in the adsorbent, while bonding to the positively charged “head” of hexadecyltrimethylammonium is responsible for modified zeolite. The adsorption isotherms corresponded well to the Polanyi-Dubinin-Manes model, indicating that pore-filling was the dominant adsorption mechanism.
Cetyltrimethylammonium bromide, a cationic surfactant with long alkyl chains, creates a suitable organic–inorganic framework for pharmaceutical compound adsorption. It was found to be suitable for synthesizing organobentonite adsorbents with high ibuprofen and diclofenac molecule adsorption capacities of 194 mg/g and 600 mg/g, respectively (Ghemit et al. 2019). The higher potential for the uptake of pharmaceutical contaminants was attributed to the larger interlayer space within organobentonites. The chemical nature of bentonite changes from hydrophilic to hydrophobic by intercalating cationic surfactants through ion exchange, and consequently, hydrophobic interactions play an essential role during adsorption. Both ibuprofen and diclofenac molecules were divided into the organic phase of the interlayer space made by the surfactant. The amount of ibuprofen and diclofenac adsorbed gradually increased with increasing surfactant concentration. In the competitive adsorption of ibuprofen and diclofenac, their monolayer adsorption decreased to 83 mg/g and 188 mg/g, respectively.
Functionalizing clays with amines was also investigated for ibuprofen adsorptive removal. For example, the amine-functionalized nano-clay Cloisite 15A was successfully used for the adsorptive removal of ibuprofen in both batches (Rafati et al. 2018) and continuous fixed-bed column (Rafati et al. 2019) systems. Rafati et al. (2019) showed that the adsorption capacity of the fixed-bed column depended on ibuprofen concentration and bed depth. The Thomas, bed-depth service time, Yoon-Nelson, and Clark mathematical models accurately predicted the breakthrough curves. The strong hydration of the inorganic counter ions in the interlayer space of expandable clay minerals made them hydrophilic and, therefore, often weak adsorbents toward hydrophobic organic compounds (Gámiz et al. 2015).
In another study, polyamidoamine dendrimer was grafted onto halloysite clay mineral (Kurczewska et al. 2020) with an intermediate 3- aminopropyltrimethoxysilane functionalization step. Electrostatic interactions between protonated amine groups on halloysite surfaces in both 3- aminopropyltrimethoxysilane functionalized and polyamidoamine dendrimer grafted halloysite, and the carboxyl groups in pharmaceutical molecules significantly affect adsorption efficiency. However, Tan et al. (2013) indicated that the holloysite-3-aminopropyltriethoxysilane surface had 25% higher ibuprofen loading than the unmodified halloysite. More reactive functional groups were provided by the polyamidoamine dendrimer for favorable adsorption toward ibuprofen and naproxen than organosilane (Kurczewska et al. 2020). Halloysite surfaces bearing covalently attached organic units demonstrated a higher affinity for pharmaceutical molecules than the unmodified mineral, and the ibuprofen and naproxen loading efficiencies increased to high adsorption capacities of 68 mg/g and 5.9 mg/g, respectively, at pH 6.
In a study conducted by Li et al. (2019), the adsorption mechanism of ibuprofen onto a zeolite/sepiolite nano-heterostructure and an organically modified sepiolite called Tetranyl® B-2MTH, namely stearyl dimethyl benzyl ammonium chloride, sepiolite was investigated. The study utilized isotherm studies to propose a pore-filling mechanism, indicating the formation of one or more adsorbed layers. Notably, the results revealed that the bonding of ibuprofen molecules on both adsorbents occurred in both horizontal and non-horizontal, temperature-dependent orientations. This suggests the presence of multi-docking and multi-molecular adsorption, respectively. At higher temperatures, specifically 60 °C, ibuprofen molecules in solution tended to aggregate primarily through dimer formation, with an approximate capture of two ibuprofen molecules. The organically modified sepiolite exhibited a higher adsorption capacity than the zeolite/sepiolite across all studied temperatures. The primary factors influencing the mechanism of ibuprofen adsorption were identified as the adsorption energies and the density of receptor sites. Recently, Njaramba et al. (2023) introduced a novel three-dimensional mesoporous aerogel by incorporating sepiolite and zirconium 1,4-dicarboxybenzene metal–organic framework UiO-66, a zirconium-based metal–organic framework, into gelatin. This innovative aerogel was proposed as a promising alternative adsorbent for efficiently removing ibuprofen and naproxen. The adsorption process was found to be exothermic, and it followed both the pseudo-second-order kinetics and the Langmuir isotherm models. The maximum sorption capacities for ibuprofen and naproxen were determined to be 10 mg/g and 8.5 mg/g, respectively.
The adsorption process onto clay is highly influenced by pH and is susceptible to changes in ionic strength. Behera et al. (2012) observed that the adsorption of ibuprofen onto clays increased as the ionic strength increased. This phenomenon was attributed to the partial neutralization of the positive charge on the surface of the adsorbent, which led to the contraction of the electric double layer due to the presence of chloride ions. Furthermore, chloride ions were found to enhance the adsorption of ibuprofen by effectively pairing their charges. This pairing mechanism reduced repulsion between ibuprofen molecules already adsorbed on the surface, thereby facilitating the adsorption of additional positively charged ibuprofen ions.
Polymer-based adsorbents
Polymeric materials have garnered significant attention as adsorbents for pharmaceutical applications, with chitosan being one of the most extensively studied polymers (Hamidon et al. 2022; Tseng et al. 2022; Yu et al. 2015; Reza et al. 2014; Show et al. 2022a). Methanol desorbed 81% and 74% of ibuprofen from biochar derived from wood apple and its steam-activated analog, respectively (Chakraborty et al. 2018a).
Perspective
The extensive use of ibuprofen and its potential health risks to the aquatic environment necessitate further research on its removal from aqueous solutions. Despite the significant progress made in the study of ibuprofen adsorption, several knowledge gaps still need to be addressed in this field. One crucial concern is the systematic investigation of ibuprofen removal from real or simulated wastewater. While numerous studies have been conducted, only a few have focused explicitly on real wastewater samples. It is crucial to consider variables such as the actual concentration range of ibuprofen, pH levels, presence of competitive pollutants, and temperature conditions that mimic real wastewater scenarios. Furthermore, most studies have been conducted on a laboratory scale, and the efficacy of these sorbents in real-world industrial-scale applications remains uncertain. The applicability of these sorbents in treating real samples, such as industrial and hospital wastewater, as well as groundwater, where higher concentrations of ibuprofen are expected, needs to be better understood.
The inability of some adsorbents to be reused poses a challenge regarding their sustainability and cost-effectiveness. Furthermore, there is a limited focus on dynamic adsorption systems in the existing literature. Therefore, it is essential to assess the capability of adsorbents to remove ibuprofen in dynamic adsorption systems, such as at the point of use, to evaluate their practical applicability. Substantial research is needed to develop low-cost, high-performance adsorbents that remove ibuprofen in wastewater treatment plants. This can open up opportunities for their application in domestic water filters and residential settings.
Additionally, the cost analysis of the adsorption process should be considered, as it is an essential factor that has often been neglected in previous investigations. Future research should also address disposing of spent adsorbents after prolonged use, focusing on environmentally sustainable and friendly techniques. Moreover, combining mechanical or biological treatment methods with adsorption systems should be studied to enhance the overall removal performance of ibuprofen in wastewater treatment processes. By addressing these areas of concern, advancements can be made in develo** and applying effective and sustainable ibuprofen removal technologies.
Conclusion
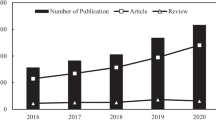
The presence of ibuprofen in wastewater has recently emerged as a significant environmental and human health concern. Among the various methods employed to remove ibuprofen, adsorption has gained significant attention, particularly in the past five years. The review has demonstrated that functionalized or modified adsorbents perform superior to pristine materials for removing ibuprofen. Various strategies, such as grafting, crosslinking, and material combinations, have been employed to enhance the adsorption capacity of these materials. The review highlighted that activated carbons and biochar are the most widely studied materials for ibuprofen removal, while metal–organic framework structures have also shown promising performance. The adsorption process was found to be influenced by the solution pH, with higher adsorption typically observed under acidic conditions, i.e., pH 3. The dominant mechanisms involved in ibuprofen adsorption were electrostatic and π-π interactions and hydrogen bonding. However, despite the progress made in understanding ibuprofen adsorption, this field is still evolving, and several aspects require continuous investigation and improvement. Essential factors such as cost, safety, and compatibility with industrial conditions must be thoroughly examined to ensure the practical applicability of these adsorbents. Further research is necessary to address these gaps and advance the development of efficient and cost-effective adsorbents for ibuprofen removal.
References
Abbasnia A, Zarei A, Yeganeh M, Sobhi HR, Gholami M, Esrafili A (2022) Removal of tetracycline antibiotics by adsorption and photocatalytic-degradation processes in aqueous solutions using metal organic frameworks (MOFs): a systematic review. Inorg Chem Commun 145:109959. https://doi.org/10.1016/j.inoche.2022.109959
Abolhasani S, Ahmadpour A, Bastami TR, Yaqubzadeh A (2019) Facile synthesis of mesoporous carbon aerogel for the removal of ibuprofen from aqueous solution by central composite experimental design (CCD). J Mol Liq 281:261–268. https://doi.org/10.1016/j.molliq.2019.02.084
Abukhadra MR, Refay NM, Nadeem A, El-Sherbeeny AM, Ibrahim KE (2020) Insight into the role of integrated carbohydrate polymers (starch, chitosan, and β-cyclodextrin) with mesoporous silica as carriers for ibuprofen drug; equilibrium and pharmacokinetic properties. Int J Biol Macromol 156:537–547. https://doi.org/10.1016/j.ijbiomac.2020.04.052
Adeleye AT, Akande AA, Odoh CK, Philip M, Fidelis TT, Amos PI, Banjoko OO (2021) Efficient synthesis of bio-based activated carbon (AC) for catalytic systems: a green and sustainable approach. J Ind Eng Chem 96:59–75. https://doi.org/10.1016/j.jiec.2021.01.044
Afifeh MR, Ahmadpour A, Mosavian MTH, Ayati A, Bamoharram FF, Hekmatikar FA (2019) A green approach to the facile synthesis of colloidal platinum nanoparticles by Preyssler polyoxometalate. J Nanostruct 9:249–257. https://doi.org/10.22052/JNS.2019.02.007
Ahmadpour A, Eftekhari N, Ayati A (2014) Performance of MWCNTs and a low-cost adsorbent for chromium(VI) ion removal. J Nanostruct Chem 4(4):171–178. https://doi.org/10.1007/s40097-014-0119-9
Ahmed MJ (2017) Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: review. J Environ Manage 190:274–282. https://doi.org/10.1016/j.jenvman.2016.12.073
Ai T, Jiang X, Zhong Z, Li D, Dai S (2020) Methanol-modified ultra-fine magnetic orange peel powder biochar as an effective adsorbent for removal of ibuprofen and sulfamethoxazole from water. Adsorp Sci Tech 38(7–8):304–321. https://doi.org/10.1177/0263617420944659
Akash S, Sivaprakash B, Rajamohan N, Govarthanan M, Elakiya BT (2022) Remediation of pharmaceutical pollutants using graphene-based materials - A review on operating conditions, mechanism and toxicology. Chemosphere 306:135520. https://doi.org/10.1016/j.chemosphere.2022.135520
Akbari Beni F, Gholami A, Ayati A, Niknam Shahrak M, Sillanpää M (2020) UV-switchable phosphotungstic acid sandwiched between ZIF-8 and Au nanoparticles to improve simultaneous adsorption and UV light photocatalysis toward tetracycline degradation. Micropor Mesopor Mater 303:110275. https://doi.org/10.1016/j.micromeso.2020.110275
Akhil D, Lakshmi D, Senthil Kumar P, Vo D-VN, Kartik A (2021) Occurrence and removal of antibiotics from industrial wastewater. Environ Chem Lett 19(2):1477–1507. https://doi.org/10.1007/s10311-020-01152-0
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383. https://doi.org/10.1016/j.jhazmat.2020.122383
Ali MEM, Abd El-Aty AM, Badawy MI, Ali RK (2018) Removal of pharmaceutical pollutants from synthetic wastewater using chemically modified biomass of green alga Scenedesmus obliquus. Ecotoxicol Environ Saf 151:144–152. https://doi.org/10.1016/j.ecoenv.2018.01.0123
Ali SNF, El-Shafey EI, Al-Busafi S, Al-Lawati HAJ (2019) Adsorption of chlorpheniramine and ibuprofen on surface functionalized activated carbons from deionized water and spiked hospital wastewater. J Environ Chem Eng 7(1):102860. https://doi.org/10.1016/j.jece.2018.102860
Alipour E, Alimohammady F, Yumashev A, Maseleno A (2019) Fullerene C60 containing porphyrin-like metal center as drug delivery system for ibuprofen drug. J Mol Model 26(1):7. https://doi.org/10.1007/s00894-019-4267-1
Alitabar-Ferozjah H, Rahbar-Kelishami A (2022) Simultaneous effect of multi-walled carbon nanotube and Span85 on the extraction of Ibuprofen from aqueous solution using emulsion liquid membrane. J Mol Liq 365:120051. https://doi.org/10.1016/j.molliq.2022.120051
Al-Khateeb LA, Al-zahrani MA, El Hamd MA, El-Maghrabey M, Dahas FA, El-Shaheny R (2021) High-temperature liquid chromatography for evaluation of the efficiency of multiwalled carbon nanotubes as nano extraction beds for removal of acidic drugs from wastewater Greenness profiling and comprehensive kinetics and thermodynamics studies. J Chromatogr A 1639:461891. https://doi.org/10.1016/j.chroma.2021.461891
Al-Kindi GY, Al-Haidri H (2021) The removal of ibuprofen drugs residues from municipal wastewater by Moringa Oleifera Seeds. J Ecol Eng 22(1):83–94. https://doi.org/10.12911/22998993/128868
Al-rimawi F, Daana M, Khamis M, Karaman R, Khoury H, Qurie M (2019) Removal of selected pharmaceuticals from aqueous solutions using natural jordanian zeolite. Arab J Sci Eng 44(1):209–215. https://doi.org/10.1007/s13369-018-3406-9
Álvarez-Torrellas S, Rodríguez A, Ovejero G, García J (2016) Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem Eng J 283:936–947. https://doi.org/10.1016/j.cej.2015.08.023
Al-Yousef HA, Alotaibi BM, Alanazi MM, Aouaini F, Sellaoui L, Bonilla-Petriciolet A (2021) Theoretical assessment of the adsorption mechanism of ibuprofen, ampicillin, orange G and malachite green on a biomass functionalized with plasma. J Environ Chem Eng 9(1):104950. https://doi.org/10.1016/j.jece.2020.104950
Al-Yousef HA, Alotaibi BM, Aouaini F, Sellaoui L, Bonilla-Petriciolet A (2021) Adsorption of ibuprofen on cocoa shell biomass-based adsorbents: interpretation of the adsorption equilibrium via statistical physics theory. J Mol Liq 331:115697. https://doi.org/10.1016/j.molliq.2021.115697
Ameen F, Mostafazadeh R, Hamidian Y, Erk N, Sanati AL, Karaman C, Ayati A (2023) Modeling of adsorptive removal of azithromycin from aquatic media by CoFe2O4/NiO anchored microalgae-derived nitrogen-doped porous activated carbon adsorbent and colorimetric quantifying of azithromycin in pharmaceutical products. Chemosphere 329:138635. https://doi.org/10.1016/j.chemosphere.2023.138635
Amiri A, Mirzaei M, Derakhshanrad S (2019) A nanohybrid composed of polyoxotungstate and graphene oxide for dispersive micro solid-phase extraction of non-steroidal anti-inflammatory drugs prior to their quantitation by HPLC. Microchim Acta 186(8):534. https://doi.org/10.1007/s00604-019-3694-0
Amrhar O, El Gana L, Mobarak M (2021) Calculation of adsorption isotherms by statistical physics models: a review. Environ Chem Lett 19(6):4519–4547. https://doi.org/10.1007/s10311-021-01279-8
An HJ, Bhadra BN, Khan NA, Jhung SH (2018) Adsorptive removal of wide range of pharmaceutical and personal care products from water by using metal azolate framework-6-derived porous carbon. Chem Eng J 343:447–454. https://doi.org/10.1016/j.cej.2018.03.025
Anand U, Adelodun B, Cabreros C, Kumar P, Suresh S, Dey A, Ballesteros F, Bontempi E (2022) Occurrence, transformation, bioaccumulation, risk and analysis of pharmaceutical and personal care products from wastewater: a review. Environ Chem Lett 20(6):3883–3904. https://doi.org/10.1007/s10311-022-01498-7
Arinkoola A, Alagbe S, Akinwole I, Ogundiran A, Ajayi L, Agbede O, Ogunleye O (2022) Adsorptive removal of Ibuprofen, Ketoprofen and Naproxen from aqueous solution using coconut shell biomass. Environ Res Eng Manage 78(2):28–37. https://doi.org/10.5755/j01.erem.78.2.29695
Aryee AA, Mpatani FM, Kani AN, Dovi E, Han R, Li Z, Qu L (2021) A review on functionalized adsorbents based on peanut husk for the sequestration of pollutants in wastewater: modification methods and adsorption study. J Clean Product 310:127502. https://doi.org/10.1016/j.jclepro.2021.127502
Ávila MI, Alonso-Morales N, Baeza JA, Rodríguez JJ, Gilarranz MA (2020) High load drug release systems based on carbon porous nanocapsule carriers Ibuprofen case study. J Mater Chem B 8(24):5293–5304. https://doi.org/10.1039/D0TB00329H
Awad H, Gar Alalm M, El-Etriby HK (2019) Environmental and cost life cycle assessment of different alternatives for improvement of wastewater treatment plants in develo** countries. Sci Total Environ 660:57–68. https://doi.org/10.1016/j.scitotenv.2018.12.386
Ayati A, Ahmadpour A, Bamoharram FF, Heravi MM, Rashidi H (2011) Photocatalytic synthesis of gold nanoparticles using preyssler acid and their photocatalytic activity. China J Catal 32(6):978–982. https://doi.org/10.1016/S1872-2067(10)60221-5
Ayati A, Shahrak MN, Tanhaei B, Sillanpää M (2016) Emerging adsorptive removal of azo dye by metal-organic frameworks. Chemosphere 160:30–44. https://doi.org/10.1016/j.chemosphere.2016.06.065
Ayati A, Ranjbari S, Tanhaei B, Sillanpää M (2019) Ionic liquid-modified composites for the adsorptive removal of emerging water contaminants: a review. J. Mol. Liq. 275:71–83. https://doi.org/10.1016/j.molliq.2018.11.016
Ayati A, Tanhaei B, Beiki H, Krivoshapkin P, Krivoshapkina E, Tracey C (2023) Insight into the adsorptive removal of ibuprofen using porous carbonaceous materials: a review. Chemosphere 323:138241. https://doi.org/10.1016/j.chemosphere.2023.138241
Baccar R, Sarrà M, Bouzid J, Feki M, Blánquez P (2012) Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem Eng J. https://doi.org/10.1016/j.cej.2012.09.099
Balakrishnan A, Appunni S, Chinthala M, Jacob MM, Vo D-VN, Reddy SS, Kunnel ES (2023) Chitosan-based beads as sustainable adsorbents for wastewater remediation: a review. Environ Chem Lett 21(3):1881–1905. https://doi.org/10.1007/s10311-023-01563-9
Bany-Aiesh H, Banat R, Al-Sou’od K (2015) Kinetics and Adsorption Isotherm of Ibuprofen onto Grafted β-CD/Chitosan Polymer. Am J Appl Sci 12(12):917–930. https://doi.org/10.3844/ajassp.2015.917.930
Barczak M (2019) Amine-modified mesoporous silicas: Morphology-controlled synthesis toward efficient removal of pharmaceuticals. Micropor Mesopor Mater 278:354–365. https://doi.org/10.1016/j.micromeso.2019.01.012
Bastami TR, Ahmadpour A, Hekmatikar FA (2017) Synthesis of Fe3O4/Bi2WO6 nanohybrid for the photocatalytic degradation of pharmaceutical ibuprofen under solar light. J Ind Eng Chem 51:244–254. https://doi.org/10.1016/j.jiec.2017.03.008
Behera SK, Oh SY, Park HS (2012) Sorptive removal of ibuprofen from water using selected soil minerals and activated carbon. Int J Environ Sci Technol 9(1):85–94. https://doi.org/10.1007/s13762-011-0020-8
Bello MM, Raman AAA (2019) Synergy of adsorption and advanced oxidation processes in recalcitrant wastewater treatment. Environ Chem Lett 17(2):1125–1142. https://doi.org/10.1007/s10311-018-00842-0
Bello OS, Alao OC, Alagbada TC, Olatunde AM (2019) Biosorption of ibuprofen using functionalized bean husks. Sustain Chem Pharm 13:100151. https://doi.org/10.1016/j.scp.2019.100151
Bello OS, Alao OC, Alagbada TC, Agboola OS, Omotoba OT, Abikoye OR (2020) A renewable, sustainable and low-cost adsorbent for ibuprofen removal. Water Sci Technol 83(1):111–122. https://doi.org/10.2166/wst.2020.551
Bello OS, Moshood MA, Ewetumo BA, Afolabi IC (2020) Ibuprofen removal using coconut husk activated Biomass. Chem Data Collect 29:100533. https://doi.org/10.1016/j.cdc.2020.100533
Benedini L, Placente D, Ruso J, Messina P (2019) Adsorption/desorption study of antibiotic and anti-inflammatory drugs onto bioactive hydroxyapatite nano-rods. Mater Sci Eng C 99:180–190. https://doi.org/10.1016/j.msec.2019.01.098
Bhadra BN, Ahmed I, Kim S, Jhung SH (2017) Adsorptive removal of ibuprofen and diclofenac from water using metal-organic framework-derived porous carbon. Chem Eng J 314:50–58. https://doi.org/10.1016/j.cej.2016.12.127
Bouissou-Schurtz C, Houeto P, Guerbet M, Bachelot M, Casellas C, Mauclaire A-C, Panetier P, Delval C, Masset D (2014) Ecological risk assessment of the presence of pharmaceutical residues in a French national water survey. Regul Toxicol Pharm 69(3):296–303. https://doi.org/10.1016/j.yrtph.2014.04.006
Brillas E (2022) A critical review on ibuprofen removal from synthetic waters, natural waters, and real wastewaters by advanced oxidation processes. Chemosphere 286:131849. https://doi.org/10.1016/j.chemosphere.2021.131849
Brun GL, Bernier M, Losier R, Doe K, Jackman P, Lee H-B (2006) Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environ Toxicol Chem 25:2163–2176. https://doi.org/10.1897/05-426r.1
Bui TX, Choi H (2009) Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J Hazard Mater 168(2):602–608. https://doi.org/10.1016/j.jhazmat.2009.02.072
Bursztyn Fuentes AL, Benito DE, Montes ML, Scian AN, Lombardi MB (2022) Paracetamol and Ibuprofen removal from aqueous phase using a ceramic-derived activated carbon. Arab J Sci Eng. https://doi.org/10.1007/s13369-022-07307-1
Caban M, Stepnowski P (2021) How to decrease pharmaceuticals in the environment? A review environmental. Chem Lett 19(4):3115–3138. https://doi.org/10.1007/s10311-021-01194-y
Cao W, Yuan Y, Yang C, Wu S, Cheng J (2020) In-situ fabrication of g-C3N4/MIL-68(In)-NH2 heterojunction composites with enhanced visible-light photocatalytic activity for degradation of ibuprofen. Chem Eng J 391:123608. https://doi.org/10.1016/j.cej.2019.123608
Chahm T, Rodrigues CA (2017) Removal of ibuprofen from aqueous solutions using O-carboxymethyl-N-laurylchitosan/γ-Fe2O3. Environ Nanotechnol Monit Manag 7:139–148. https://doi.org/10.1016/j.enmm.2017.03.0013
Chakraborty P, Banerjee S, Kumar S, Sadhukhan S, Halder G (2018) Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: Isotherm, kinetics, thermodynamics and cost estimation. Proc Safe Environ Protect 118:10–23. https://doi.org/10.1016/j.psep.2018.06.015
Chakraborty P, Show S, Banerjee S, Halder G (2018) Mechanistic insight into sorptive elimination of ibuprofen employing bi-directional activated biochar from sugarcane bagasse: performance evaluation and cost estimation. J Environ Chem Eng 6(4):5287–5300. https://doi.org/10.1016/j.jece.2018.08.017
Chakraborty P, Show S, Ur Rahman W, Halder G (2019) Linearity and non-linearity analysis of isotherms and kinetics for ibuprofen remotion using superheated steam and acid modified biochar. Proc Safe Environ Protect 126:193–204. https://doi.org/10.1016/j.psep.2019.04.011
Chakraborty P, Singh SD, Gorai I, Singh D, Rahman W-U, Halder G (2020) Explication of physically and chemically treated date stone biochar for sorptive remotion of ibuprofen from aqueous solution. J Water Proc Eng 33:101022. https://doi.org/10.1016/j.jwpe.2019.101022
Chandrashekar KS, Balakrishnan RM (2021) Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. J Clean Product 294:126155. https://doi.org/10.1016/j.jclepro.2021.126155
Chang F, Memon N, Memon S, Chang AS (2022) Selective adsorption of emerging contaminants from aqueous solution using Cu-based composite by solvothermal. Int J Environ Sci Technol 19(11):11161–11168. https://doi.org/10.1007/s13762-021-03882-2
Chávez AM, Rey A, López J, Álvarez PM, Beltrán FJ (2021) Critical aspects of the stability and catalytic activity of MIL-100(Fe) in different advanced oxidation processes. Sep Pur Technol 255:117660. https://doi.org/10.1016/j.seppur.2020.117660
Chen C, Xu L, Huo J-B, Gupta K, Fu M-L (2020) Simultaneous removal of butylparaben and arsenite by MOF-derived porous carbon coated lanthanum oxide: combination of persulfate activation and adsorption. Chem. Eng. J 391:123552. https://doi.org/10.1016/j.cej.2019.123552
Cho H-H, Huang H, Schwab K (2011) Effects of solution chemistry on the adsorption of Ibuprofen and Triclosan onto carbon nanotubes. Langmuir 27(21):12960–12967. https://doi.org/10.1021/la202459g
Choi J, Shin WS (2020) Removal of Salicylic and Ibuprofen by Hexadecyltrimethylammonium-Modified Montmorillonite and Zeolite. Minerals 10(10):898. https://doi.org/10.3390/min10100898
Choi M, Choi DW, Lee JY, Kim YS, Kim BS, Lee BH (2012) Removal of pharmaceutical residue in municipal wastewater by DAF (dissolved air flotation)–MBR (membrane bioreactor) and ozone oxidation. Water Sci Technol 66(12):2546–2555. https://doi.org/10.2166/wst.2012.429
Choong CE, Ibrahim S, Basirun WJ (2019) Mesoporous silica from batik sludge impregnated with aluminum hydroxide for the removal of bisphenol A and ibuprofen. J Colloid Interf Sci 541:12–17. https://doi.org/10.1016/j.jcis.2019.01.071
Chopra S, Kumar D (2020) Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 6(6):e04087. https://doi.org/10.1016/j.heliyon.2020.e04087
Choudhary V, Philip L (2022) Sustainability assessment of acid-modified biochar as adsorbent for the removal of pharmaceuticals and personal care products from secondary treated wastewater. J Environ Chem Eng 10(3):107592. https://doi.org/10.1016/j.jece.2022.107592
Ciesielczyk F, Goscianska J, Zdarta J, Jesionowski T (2018) The development of zirconia/silica hybrids for the adsorption and controlled release of active pharmaceutical ingredients. Coll Surf A: Physicochem Eng Asp 545:39–50. https://doi.org/10.1016/j.colsurfa.2018.02.036
Ciesielczyk F, Żółtowska-Aksamitowska S, Jankowska K, Zembrzuska J, Zdarta J, Jesionowski T (2019) The role of novel lignosulfonate-based sorbent in a sorption mechanism of active pharmaceutical ingredient: batch adsorption tests and interaction study. Adsorption 25(4):865–880. https://doi.org/10.1007/s10450-019-00099-1
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142(3):185–194. https://doi.org/10.1016/S0378-4274(03)00068-7
Coimbra RN, Calisto V, Ferreira CIA, Esteves VI, Otero M (2019) Removal of pharmaceuticals from municipal wastewater by adsorption onto pyrolyzed pulp mill sludge. Arab J Chem 12(8):3611–3620. https://doi.org/10.1016/j.arabjc.2015.12.001
Collado N, Buttiglieri G, Ferrando-Climent L, Rodriguez-Mozaz S, Barceló D, Comas J, Rodriguez-Roda I (2012) Removal of ibuprofen and its transformation products: experimental and simulation studies. Sci Total Environ. 433:296–301. https://doi.org/10.1016/j.scitotenv.2012.06.060
Davarnejad R, Soofi B, Farghadani F, Behfar R (2018) Ibuprofen removal from a medicinal effluent: a review on the various techniques for medicinal effluents treatment. Environ Technol Innov 11:308–320. https://doi.org/10.1016/j.eti.2018.06.011
de Boer MA, Hammerton M, Slootweg JC (2018) Uptake of pharmaceuticals by sorbent-amended struvite fertilisers recovered from human urine and their bioaccumulation in tomato fruit. Water Res 133:19–26. https://doi.org/10.1016/j.watres.2018.01.017
de Melo Pirete L, Camargo FP, Dornelles HS, Granatto CF, Sakamoto IK, Grosseli GM, Fadini PS, Silva EL, Varesche MBA (2022) Biodegradation of diclofenac and ibuprofen in Fluidized Bed Reactor applied to sanitary sewage treatment in acidogenic and denitrifying conditions. J Water Proc Eng 49:102964. https://doi.org/10.1016/j.jwpe.2022.102964
De Oliveira T, Boussafir M, Fougère L, Destandau E, Sugahara Y, Guégan R (2020) Use of a clay mineral and its nonionic and cationic organoclay derivatives for the removal of pharmaceuticals from rural wastewater effluents. Chemosphere 259:127480. https://doi.org/10.1016/j.chemosphere.2020.127480
Delgado-Moreno L, Bazhari S, Gasco G, Méndez A, El Azzouzi M, Romero E (2021) New insights into the efficient removal of emerging contaminants by biochars and hydrochars derived from olive oil wastes. Sci Total Environ 752:141838. https://doi.org/10.1016/j.scitotenv.2020.141838
Delle Piane M, Vaccari S, Corno M, Ugliengo P (2014) Silica-based materials as drug adsorbents: first principle investigation on the role of water microsolvation on ibuprofen adsorption. J Phys Chem A 118(31):5801–5807. https://doi.org/10.1021/jp411173k
Diagboya PNE, Dikio ED (2018) Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Micropor Mesopor Mater 266:252–267. https://doi.org/10.1016/j.micromeso.2018.03.008
Du Y-d, Zhang X-q, Shu L, Feng Y, Lv C, Liu H-q, Xu F, Wang Q, Zhao C-c, Kong Q (2021) Safety evaluation and ibuprofen removal via an Alternanthera philoxeroides-based biochar. Environ Sci Pollut Res 28(30):40568–40586. https://doi.org/10.1007/s11356-020-09714-z
Duarte EDV, Oliveira MG, Spaolonzi MP, Costa HPS, Silva TLd, Silva MGCd, Vieira MGA (2022) Adsorption of pharmaceutical products from aqueous solutions on functionalized carbon nanotubes by conventional and green methods: a critical review. J Clean Product. 372:133743. https://doi.org/10.1016/j.jclepro.2022.133743
Dubey SP, Dwivedi AD, Sillanpää M, Gopal K (2010) Artemisia vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem Eng J 165(2):537–544. https://doi.org/10.1016/j.cej.2010.09.068
Eizi R, Bastami TR, Mahmoudi V, Ayati A, Babaei H (2023) Facile ultrasound-assisted synthesis of CuFe-Layered double hydroxides/g-C3N4 nanocomposite for alizarin red S sono-sorption. J Taiwan Instit Chem Eng 145:104844. https://doi.org/10.1016/j.jtice.2023.104844
Ejeromedoghene O, Oderinde O, Okoye CO, Oladipo A, Alli YA (2022) Microporous metal-organic frameworks based on deep eutectic solvents for adsorption of toxic gases and volatile organic compounds: a review. Chem Eng J Adv 12:100361. https://doi.org/10.1016/j.ceja.2022.100361
Elizalde-Velázquez A, Subbiah S, Anderson TA, Green MJ, Zhao X, Cañas-Carrell JE (2020) Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci Total Environ. 715:136974. https://doi.org/10.1016/j.scitotenv.2020.136974
El-Sheikh AH, Qawariq RF, Abdelghani JI (2019) Adsorption and magnetic solid-phase extraction of NSAIDs from pharmaceutical wastewater using magnetic carbon nanotubes: effect of sorbent dimensions, magnetite loading and competitive adsorption study. Environ Technol Innov 16:100496. https://doi.org/10.1016/j.eti.2019.100496
Essandoh M, Kunwar B, Pittman CU, Mohan D, Mlsna T (2015) Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem Eng J 265:219–227. https://doi.org/10.1016/j.cej.2014.12.006
Estevez E, Hernandez-Moreno JM, Fernandez-Vera JR, Palacios-Diaz MP (2014) Ibuprofen adsorption in four agricultural volcanic soils. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2013.07.068
Fang T-H, Nan F-H, Chin T-S, Feng H-M (2012) The occurrence and distribution of pharmaceutical compounds in the effluents of a major sewage treatment plant in Northern Taiwan and the receiving coastal waters. Mar Pollut Bull 64(7):1435–1444. https://doi.org/10.1016/j.marpolbul.2012.04.008
Farghali M, Mohamed IMA, Osman AI, Rooney DW (2023) Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review. Environ Chem Lett 21(1):97–152. https://doi.org/10.1007/s10311-022-01520-y
Farrokhi Z, Ayati A, Kanvisi M, Sillanpää M (2019) Recent advance in antibacterial activity of nanoparticles contained polyurethane. J Appl Polym Sci 136:46997. https://doi.org/10.1002/app.46997
Fayyazi M, Solaimany Nazar AR, Farhadian M, Tangestaninejad S (2022) Adsorptive removal of ibuprofen to binary and amine-functionalized UiO-66 in the aquatic environment: synergistic/antagonistic evaluation. Environ Sci Pollut Res 29(46):69502–69516. https://doi.org/10.1007/s11356-022-20703-2
Femina Carolin C, Senthil Kumar P, Janet Joshiba G, Vinoth Kumar V (2021) Analysis and removal of pharmaceutical residues from wastewater using membrane bioreactors: a review. Environ Chem Lett 19(1):329–343. https://doi.org/10.1007/s10311-020-01068-9
Ferrah N, Merghache D, Meftah S, Benbellil S (2022) A new alternative of a green polymeric matrix chitosan/alginate-polyethyleniminemethylene phosphonic acid for pharmaceutical residues adsorption. Environ Sci Pollut Res 29(9):13675–13687. https://doi.org/10.1007/s11356-021-16599-z3
Ferrer-Polonio E, Fernández-Navarro J, Iborra-Clar M-I, Alcaina-Miranda M-I, Mendoza-Roca JA (2020) Removal of pharmaceutical compounds commonly-found in wastewater through a hybrid biological and adsorption process. J Environ Manage 263:110368. https://doi.org/10.1016/j.jenvman.2020.110368
Franco DSP, Pinto D, Georgin J, Netto MS, Foletto EL, Manera C, Godinho M, Silva LFO, Dotto GL (2022) Conversion of Erythrina speciosa pods to porous adsorbent for Ibuprofen removal. J Environ Chem Eng 10(3):108070. https://doi.org/10.1016/j.jece.2022.108070
Fröhlich AC, dos Reis GS, Pavan FA, Lima ÉC, Foletto EL, Dotto GL (2018) Improvement of activated carbon characteristics by sonication and its application for pharmaceutical contaminant adsorption. Environ Sci Pollut Res 25(25):24713–24725. https://doi.org/10.1007/s11356-018-2525-x
Fröhlich AC, Ocampo-Pérez R, Diaz-Blancas V, Salau NPG, Dotto GL (2018) Three-dimensional mass transfer modeling of ibuprofen adsorption on activated carbon prepared by sonication. Chem Eng J 341:65–74. https://doi.org/10.1016/j.cej.2018.02.020
Fröhlich AC, Foletto EL, Dotto GL (2019) Preparation and characterization of NiFe2O4/activated carbon composite as potential magnetic adsorbent for removal of ibuprofen and ketoprofen pharmaceuticals from aqueous solutions. J Clean Product 229:828–837. https://doi.org/10.1016/j.jclepro.2019.05.037
Gámiz B, Hermosín MC, Cornejo J, Celis R (2015) Hexadimethrine-montmorillonite nanocomposite: characterization and application as a pesticide adsorbent. Appl Surf Sci 332:606–613. https://doi.org/10.1016/j.apsusc.2015.01.179
Ganesan S, Eswaran M, Chokkiah B, Dhanusuraman R, Lingassamy AP, Ponnusamy VK, Kumar G, Pugazhendhi A (2021) Facile and low-cost production of Lantana camara stalk-derived porous carbon nanostructures with excellent supercapacitance and adsorption performance. Int J. Energy Res 45(12):17440–17449. https://doi.org/10.1002/er.5730
Geiger E, Hornek-Gausterer R, Saçan MT (2016) Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxic Environ Saf 129:189–198. https://doi.org/10.1016/j.ecoenv.2016.03.032
Gellerstedt G, Henriksson G (2008) Chapter 9 - Lignins: major sources, structure and properties. In: Belgacem MN, Gandini A (eds) Monomers, polymers and composites from renewable resources. Elsevier, Amsterdam, pp 201–224. https://doi.org/10.1016/B978-0-08-045316-3.00009-0
Gennaro Bd, Izzo F, Catalanotti L, Langella A, Mercurio M (2017) Surface modified phillipsite as a potential carrier for NSAIDs release. Adv Sci Lett 23(6):5941–5943. https://doi.org/10.1166/asl.2017.9075
Ghasemi M, Khedri M, Didandeh M, Taheri M, Ghasemy E, Maleki R, Shon Hk, Razmjou A (2022) Removal of pharmaceutical pollutants from wastewater using 2D covalent organic frameworks (COFs): an in silico engineering study. Ind Eng Chem Res 61(25):8809–8820. https://doi.org/10.1021/acs.iecr.2c00924
Ghemit R, Makhloufi A, Djebri N, Flilissa A, Zerroual L, Boutahala M (2019) Adsorptive removal of diclofenac and ibuprofen from aqueous solution by organobentonites: study in single and binary systems. Groundw Sustain Dev 8:520–529. https://doi.org/10.1016/j.gsd.2019.02.004
Gibson R, Durán-Álvarez JC, Estrada KL, Chávez A, Jiménez Cisneros B (2010) Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 81(11):1437–1445. https://doi.org/10.1016/j.chemosphere.2010.09.006
González-Hurtado M, Marins JA, Soares BG, Briones JR, Rodríguez AR, Ortiz-Islas E (2018) Magnetic SiO2-Fe3O4 nanocomposites as carriers of Ibuprofen for controlled release applications. Rev Adv Mater Sci 55(1):12–20. https://doi.org/10.1515/rams-2018-0023
Gopinath A, Kadirvelu K (2018) Strategies to design modified activated carbon fibers for the decontamination of water and air. Environ Chem Lett 16(4):1137–1168. https://doi.org/10.1007/s10311-018-0740-9
Gopinath KP, Vo D-VN, Gnana Prakash D, Adithya Joseph A, Viswanathan S, Arun J (2021) Environmental applications of carbon-based materials: a review. Environ Chem Lett 19(1):557–582. https://doi.org/10.1007/s10311-020-01084-9
Gothwal R, Shashidhar T (2015) Antibiotic pollution in the environment: a review. CLEAN–Soil Air Water 43(4):479–489. https://doi.org/10.1002/clen.201300989
Grzesiuk M, Pijanowska J, Markowska M, Bednarska A (2020) Morphological deformation of Daphnia magna embryos caused by prolonged exposure to ibuprofen. Environ Pollut 261:114135. https://doi.org/10.1016/j.envpol.2020.114135
Gu Y, Huang J, Zeng G, Shi L, Shi Y, Yi K (2018) Fate of pharmaceuticals during membrane bioreactor treatment: status and perspectives. Bioresour Technol 268:733–748. https://doi.org/10.1016/j.biortech.2018.08.029
Guedidi H, Reinert L, Lévêque J-M, Soneda Y, Bellakhal N, Duclaux L (2013) The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon 54:432–443. https://doi.org/10.1016/j.carbon.2012.11.059
Guillossou R, Le Roux J, Mailler R, Vulliet E, Morlay C, Nauleau F, Gasperi J, Rocher V (2019) Organic micropollutants in a large wastewater treatment plant: what are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 218:1050–1060. https://doi.org/10.1016/j.chemosphere.2018.11.182
Guillossou R, Le Roux J, Mailler R, Pereira-Derome CS, Varrault G, Bressy A, Vulliet E, Morlay C, Nauleau F, Rocher V, Gasperi J (2020) Influence of dissolved organic matter on the removal of 12 organic micropollutants from wastewater effluent by powdered activated carbon adsorption. Water Res 172:115487. https://doi.org/10.1016/j.watres.2020.115487
Gul A, Khaligh NG, Julkapli NM (2021) Surface modification of carbon-based nanoadsorbents for the advanced wastewater treatment. J Mol Struct 1235:130148. https://doi.org/10.1016/j.molstruc.2021.130148
Gutiérrez-Noya VM, Gómez-Oliván LM, Ramírez-Montero MdC, Islas-Flores H, Galar-Martínez M, Dublán-García O, Romero R (2020) Ibuprofen at environmentally relevant concentrations alters embryonic development, induces teratogenesis and oxidative stress in Cyprinus carpio. Sci Total Environ. 710:136327. https://doi.org/10.1016/j.scitotenv.2019.136327
Hamidon TS, Adnan R, Haafiz MKM, Hussin MH (2022) Cellulose-based beads for the adsorptive removal of wastewater effluents: a review. Environ Chem Lett 20(3):1965–2017. https://doi.org/10.1007/s10311-022-01401-4
Hanbali G, Jodeh S, Hamed O, Bol R, Khalaf B, Qdemat A, Samhan S (2020) Enhanced Ibuprofen adsorption and desorption on synthesized functionalized magnetic multiwall carbon nanotubes from aqueous solution. Mater (Basel). https://doi.org/10.3390/ma13153329
Hasan Z, Choi E-J, Jhung SH (2013) Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem Eng J 219:537–544. https://doi.org/10.1016/j.cej.2013.01.002
Hasan M, Alfredo K, Murthy S, Riffat R (2021) Biodegradation of salicylic acid, acetaminophen and ibuprofen by bacteria collected from a full-scale drinking water biofilter. J Enviorn Manage 295:113071. https://doi.org/10.1016/j.jenvman.2021.113071
Haydar S, Ferro-Garcia MA, Rivera-Utrilla J, Joly JP (2003) Adsorption of p-nitrophenol on an activated carbon with different oxidations. Carbon 41(3):387–395. https://doi.org/10.1016/S0008-6223(02)00344-5
Horcajada P, Serre C, Maurin G, Ramsahye NA, Balas F, Vallet-Regí M, Sebban M, Taulelle F, Férey G (2008) Flexible porous Metal-Organic Frameworks for a controlled drug delivery. J Am Chem Soc 130(21):6774–6780. https://doi.org/10.1021/ja710973k
Hounfodji JW, Kanhounnon WG, Kpotin G, Atohoun GS, Lainé J, Foucaud Y, Badawi M (2021) Molecular insights on the adsorption of some pharmaceutical residues from wastewater on kaolinite surfaces. Chem Eng J 407:127176. https://doi.org/10.1016/j.cej.2020.127176
Huang L, Shen R, Shuai Q (2021) Adsorptive removal of pharmaceuticals from water using metal-organic frameworks: A review. J Environ Manage 277:111389. https://doi.org/10.1016/j.jenvman.2020.111389
Huppert N, Würtele M, Hahn HH (1998) Determination of the plasticizer N-butylbenzenesulfonamide and the pharmaceutical Ibuprofen in wastewater using solid phase microextraction (SPME). Fresenius’ J Anal Chem 362(6):529–536. https://doi.org/10.1007/s002160051119
Huxford RC, Della Rocca J, Lin W (2010) Metal–organic frameworks as potential drug carriers. Curr Opin Chem Biol 14(2):262–268. https://doi.org/10.1016/j.cbpa.2009.12.012
Igwegbe CA, Oba SN, Aniagor CO, Adeniyi AG, Ighalo JO (2021) Adsorption of ciprofloxacin from water: a comprehensive review. J Ind Eng Chem 93:57–77. https://doi.org/10.1016/j.jiec.2020.09.023
Izzo F, Mercurio M, de Gennaro B, Aprea P, Cappelletti P, Daković A, Germinario C, Grifa C, Smiljanic D, Langella A (2019) Surface modified natural zeolites (SMNZs) as nanocomposite versatile materials for health and environment. Coll Surf B 182:110380. https://doi.org/10.1016/j.colsurfb.2019.110380
Jadach B, Feliczak-Guzik A, Nowak I, Milanowski B, Piotrowska-Kempisty H, Murias M, Lulek J (2019) Modifying release of poorly soluble active pharmaceutical ingredients with the amine functionalized SBA-16 type mesoporous materials. J Biomater Appl 33(9):1214–1231. https://doi.org/10.1177/0885328219830823
Jain M, Khan SA, Pandey A, Pant KK, Ziora ZM, Blaskovich MAT (2021) Instructive analysis of engineered carbon materials for potential application in water and wastewater treatment. Sci Total Environ 793:148583. https://doi.org/10.1016/j.scitotenv.2021.148583
Jamil S, Loganathan P, Kandasamy J, Listowski A, McDonald JA, Khan SJ, Vigneswaran S (2020) Removal of organic matter from wastewater reverse osmosis concentrate using granular activated carbon and anion exchange resin adsorbent columns in sequence. Chemosphere 261:127549. https://doi.org/10.1016/j.chemosphere.2020.127549
Jean-Rameaux B, Brice T, Sadou D, Jean-Baptiste T, Berthelot ST, Elie A, Georges KY, Samuel L (2021) Multi-functionalized cellulosic biomass by plasma-assisted bonding of α-amino carboxylic acid to enhance the removal of ibuprofen in aqueous solution. J. Polym. Environ. 29(4):1176–1191. https://doi.org/10.1007/s10924-020-01958-7
Jian N, Qian L, Wang C, Li R, Xu Q, Li J (2019) Novel nanofibers mat as an efficient, fast and reusable adsorbent for solid phase extraction of non-steroidal anti-inflammatory drugs in environmental water. J Hazard Mater 363:81–89. https://doi.org/10.1016/j.jhazmat.2018.09.052
Jiang M, Yang W, Zhang Z, Yang Z, Wang Y (2015) Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. J Environ Sci 31:226–234. https://doi.org/10.1016/j.jes.2014.09.035
** X, Zhang S, Yang S, Zong Y, Xu L, ** P, Yang C, Hu S, Li Y, Shi X, Wang XC (2021) Behaviour of ozone in the hybrid ozonation-coagulation (HOC) process for ibuprofen removal: reaction selectivity and effects on coagulant hydrolysis. Sci Total Environ 794:148685. https://doi.org/10.1016/j.scitotenv.2021.148685
Jun B-M, Heo J, Park CM, Yoon Y (2019) Comprehensive evaluation of the removal mechanism of carbamazepine and ibuprofen by metal organic framework. Chemosphere 235:527–537. https://doi.org/10.1016/j.chemosphere.2019.06.208
Jung C, Son A, Her N, Zoh K-D, Cho J, Yoon Y (2015) Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: a review. J Ind Eng Chem 27:1–11. https://doi.org/10.1016/j.jiec.2014.12.035
Kamarudin NHN, Jalil AA, Triwahyono S, Salleh NFM, Karim AH, Mukti RR, Hameed BH, Ahmad A (2013) Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: effect on physicochemical properties. Micropor Mesopor Mater 180:235–241. https://doi.org/10.1016/j.micromeso.2013.06.041
Kamarudin NHN, Jalil AA, Triwahyono S, Sazegar MR, Hamdan S, Baba S, Ahmad A (2015) Elucidation of acid strength effect on ibuprofen adsorption and release by aluminated mesoporous silica nanoparticles. RSC Adv. 5(38):30023–30031. https://doi.org/10.1039/C4RA16761A
Kanakaraju D, Glass BD, Oelgemöller M (2014) Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ Chem Lett 12(1):27–47. https://doi.org/10.1007/s10311-013-0428-0
Karimi F, Ayati A, Tanhaei B, Sanati AL, Afshar S, Kardan A, Dabirifar Z, Karaman C (2022) Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent: a powerful approach in water treatment. Environ Res 203:111753. https://doi.org/10.1016/j.envres.2021.111753
Karimi-Maleh H, Ayati A, Davoodi R, Tanhaei B, Karimi F, Malekmohammadi S, Orooji Y, Fu L, Sillanpää M (2021) Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: a review. J Clean Product 291:125880. https://doi.org/10.1016/j.jclepro.2021.125880
Karimi-Maleh H, Orooji Y, Karimi F, Alizadeh M, Baghayeri M, Rouhi J, Tajik S, Beitollahi H, Agarwal S, Gupta VK, Rajendran S, Ayati A, Fu L, Sanati AL, Tanhaei B, Sen F, Shabani-nooshabadi M, Asrami PN, Al-Othman A (2021) A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens Bioelec 184:113252. https://doi.org/10.1016/j.bios.2021.113252
Karimi-Maleh H, Ranjbari S, Tanhaei B, Ayati A, Orooji Y, Alizadeh M, Karimi F, Salmanpour S, Rouhi J, Sillanpää M, Sen F (2021) Novel 1-butyl-3-methylimidazolium bromide impregnated chitosan hydrogel beads nanostructure as an efficient nanobio-adsorbent for cationic dye removal: Kinetic study. Environ Res 195:110809. https://doi.org/10.1016/j.envres.2021.110809
Kaur H, Bansiwal A, Hippargi G, Pophali GR (2018) Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: adsorption isotherms, kinetics and mechanism. Environ Sci Pollut Res Int 25(21):20473–20485. https://doi.org/10.1007/s11356-017-0054-7
Kebede TG, Dube S, Nindi MM (2019) Biopolymer electrospun nanofibres for the adsorption of pharmaceuticals from water systems. J Environ Chem Eng 7(5):103330. https://doi.org/10.1016/j.jece.2019.103330
Kermia AEB, Fouial-Djebbar D, Trari M (2016) Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus Chimie 19(8):963–970. https://doi.org/10.1016/j.crci.2016.05.005
Khadir A, Motamedi M, Negarestani M, Sillanpää M, Sasani M (2020) Preparation of a nano bio-composite based on cellulosic biomass and conducting polymeric nanoparticles for ibuprofen removal: kinetics, isotherms, and energy site distribution. Int J Biol Macromol 162:663–677. https://doi.org/10.1016/j.ijbiomac.2020.06.095
Khalaf S, Al-Rimawi F, Khamis M, Zimmerman D, Shuali U, Nir S, Scrano L, Bufo SA, Karaman R (2013) Efficiency of advanced wastewater treatment plant system and laboratory-scale micelle-clay filtration for the removal of ibuprofen residues. J Environ Sci Health Part B 48(9):814–821. https://doi.org/10.1080/03601234.2013.781372
Khalil AME, Memon FA, Tabish TA, Salmon D, Zhang S, Butler D (2020) Nanostructured porous graphene for efficient removal of emerging contaminants (pharmaceuticals) from water. Chem Eng J 398:125440. https://doi.org/10.1016/j.cej.2020.125440
Khalil AME, Memon FA, Tabish TA, Fenton B, Salmon D, Zhang S, Butler D (2021) Performance evaluation of porous graphene as filter media for the removal of pharmaceutical/emerging contaminants from water and wastewater. Nanomater (Basel). https://doi.org/10.3390/nano11010079
Khoshkho SM, Tanhaei B, Ayati A, Kazemi M (2021) Preparation and characterization of ionic and non-ionic surfactants impregnated κ-carrageenan hydrogel beads for investigation of the adsorptive mechanism of cationic dye to develop for biomedical applications. J Mol Liq 324:115118. https://doi.org/10.1016/j.molliq.2020.115118
Kim S, Park CM, Jang A, Jang M, Hernández-Maldonado AJ, Yu M, Heo J, Yoon Y (2019) Removal of selected pharmaceuticals in an ultrafiltration-activated biochar hybrid system. J Memb Sci. https://doi.org/10.1016/j.memsci.2018.10.036
Kittappa S, Jang M, Ramalingam M, Ibrahim S (2020) Amine functionalized magnetic nano-composite materials for the removal of selected endocrine disrupting compounds and its mechanism study. J Environ Chem Eng 8(4):103839. https://doi.org/10.1016/j.jece.2020.103839
Kumar L, Ragunathan V, Chugh M, Bharadvaja N (2021) Nanomaterials for remediation of contaminants: a review. Environ Chem Lett 19(4):3139–3163. https://doi.org/10.1007/s10311-021-01212-z5
Kumar VVPN, Rajendran HK, Ray J, Narayanasamy S (2022) Sequestration and toxicological assessment of emerging contaminants with polypyrrole modified carboxymethyl cellulose (CMC/PPY): case of ibuprofen pharmaceutical drug. Int J Biol Macromol 221:547–557. https://doi.org/10.1016/j.ijbiomac.2022.09.046
Kurczewska J, Cegłowski M, Schroeder G (2020) PAMAM-halloysite Dunino hybrid as an effective adsorbent of ibuprofen and naproxen from aqueous solutions. Appl Clay Sci 190:105603. https://doi.org/10.1016/j.clay.2020.105603
Kusmierek K, Dabek L, Swiatkowski A (2020) Comparative study on the adsorption kinetics and equilibrium of common water contaminants onto bentonite. Desal Water Treat 186:373–381. https://doi.org/10.5004/dwt.2020.25476
Labuto G, Carvalho AP, Mestre AS, dos Santos MS, Modesto HR, Martins TD, Lemos SG, da Silva HDT, Carrilho ENVM, Carvalho WA (2022) Individual and competitive adsorption of ibuprofen and caffeine from primary sewage effluent by yeast-based activated carbon and magnetic carbon nanocomposite. Sustain Chem Pharm 28:100703. https://doi.org/10.1016/j.scp.2022.100703
Lee G, Yoo DK, Ahmed I, Lee HJ, Jhung SH (2023) Metal-organic frameworks composed of nitro groups: preparation and applications in adsorption and catalysis. Chem Eng J 451:138538. https://doi.org/10.1016/j.cej.2022.138538
Lestari WW, Arvinawati M, Martien R, Kusumaningsih T (2018) Green and facile synthesis of MOF and nano MOF containing zinc(II) and benzen 1,3,5-tri carboxylate and its study in Ibuprofen slow-release. Mater Chem Phys 204:141–146. https://doi.org/10.1016/j.matchemphys.2017.10.034
Lestari WW, Tedra RA, Rosari VA, Saraswati TE (2020) The novel composite material MOF-[Mg3(BTC)2]/GO/Fe3O4 and its use in slow-release ibuprofen. Appl Organometal Chem 34(8):e5670. https://doi.org/10.1002/aoc.5670
Li SQ, Zhang XD, Huang YM (2017) Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J Hazard Mater 321:711–719. https://doi.org/10.1016/j.jhazmat.2016.09.065
Li Z, Gómez-Avilés A, Sellaoui L, Bedia J, Bonilla-Petriciolet A, Belver C (2019) Adsorption of ibuprofen on organo-sepiolite and on zeolite/sepiolite heterostructure: synthesis, characterization and statistical physics modeling. Chem Eng J 371:868–875. https://doi.org/10.1016/j.cej.2019.04.138
Liang Y, Feng L, Liu X, Zhao Y, Chen Q, Sui Z, Wang N (2021) Enhanced selective adsorption of NSAIDs by covalent organic frameworks via functional group tuning. Chem Eng J 404:127095. https://doi.org/10.1016/j.cej.2020.127095
Liao R, Li M, Li W, Lin X, Liu D, Wang L (2018) Efficient absorption of ibuprofen in aqueous solution using eco-friendly C3N4/soot composite. J Mater Sci 53(8):5929–5941. https://doi.org/10.1007/s10853-017-1963-z
Lin S, Zhao Y, Yun Y-S (2018) Highly effective removal of nonsteroidal anti-inflammatory pharmaceuticals from water by Zr(IV)-based metal-organic framework: adsorption performance and mechanisms. ACS Appl Mater Interfaces 10(33):28076–28085. https://doi.org/10.1021/acsami.8b08596
Lin L, Jiang W, Bechelany M, Nasr M, Jarvis J, Schaub T, Sapkota RR, Miele P, Wang H, Xu P (2019) Adsorption and photocatalytic oxidation of ibuprofen using nanocomposites of TiO2 nanofibers combined with BN nanosheets: degradation products and mechanisms. Chemosphere 220:921–929. https://doi.org/10.1016/j.chemosphere.2018.12.184
Liu Y, Liu R, Li M, Yu F, He C (2019) Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohyd Polym 220:141–148. https://doi.org/10.1016/j.carbpol.2019.05.060
Liu D, Gu W, Zhou L, Wang L, Zhang J, Liu Y, Lei J (2022) Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem Eng J 427:131503. https://doi.org/10.1016/j.cej.2021.131503
Liyanage AS, Canaday S, Pittman CU, Mlsna T (2020) Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas fir biochar adsorbents. Chemosphere 258:127336. https://doi.org/10.1016/j.chemosphere.2020.127336
López YC, Viltres H, Gupta NK, Acevedo-Peña P, Leyva C, Ghaffari Y, Gupta A, Kim S, Bae J, Kim KS (2021) Transition metal-based metal–organic frameworks for environmental applications: a review. Environ Chem Lett 19(2):1295–1334. https://doi.org/10.1007/s10311-020-01119-1
Lotfi R, Hayati B, Rahimi S, Shekarchi AA, Mahmoodi NM, Bagheri A (2019) Synthesis and characterization of PAMAM/SiO2 nanohybrid as a new promising adsorbent for pharmaceuticals. Microchem J 146:1150–1159. https://doi.org/10.1016/j.microc.2019.02.048
Lou Y, Tan FJ, Zeng R, Wang M, Li P, **a S (2020) Preparation of cross-linked graphene oxide on polyethersulfone membrane for pharmaceuticals and personal care products removal. Polym. 12(9):1921. https://doi.org/10.3390/polym12091921
Lung I, Soran M-L, Stegarescu A, Opris O, Gutoiu S, Leostean C, Lazar MD, Kacso I, Silipas T-D, Porav AS (2021) Evaluation of CNT-COOH/MnO2/Fe3O4 nanocomposite for ibuprofen and paracetamol removal from aqueous solutions. J Hazard Mater 403:123528. https://doi.org/10.1016/j.jhazmat.2020.123528
Luo R, Li X, Xu H, Sun Y, Wu J (2020) Effects of temperature, solution pH, and ball-milling modification on the adsorption of non-steroidal anti-inflammatory drugs onto biochar. Bull Environ Contamin Toxic 105(3):422–427. https://doi.org/10.1007/s00128-020-02948-0
Ma L-J, Wang J, Han M, Jia J, Wu H-S, Zhang X (2019) Adsorption of multiple H2 molecules on the complex TiC6H6: an unusual combination of chemisorption and physisorption. Energy 171:315–325. https://doi.org/10.1016/j.energy.2019.01.018
Madhura L, Singh S, Kanchi S, Sabela M, Bisetty K, Inamuddin, (2019) Nanotechnology-based water quality management for wastewater treatment. Environ Chem Lett 17(1):65–121. https://doi.org/10.1007/s10311-018-0778-8
Madikizela LM, Chimuka L (2017) Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ Monit Assess 189(7):348. https://doi.org/10.1007/s10661-017-6069-1
Madima N, Mishra SB, Inamuddin I, Mishra AK (2020) Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater A review. Environ Chem Lett 18(4):1169–1191. https://doi.org/10.1007/s10311-020-01001-0
Magadini DL, Goes JI, Ortiz S, Lipscomb J, Pitiranggon M, Yan B (2020) Assessing the sorption of pharmaceuticals to microplastics through in-situ experiments in New York City waterways. Sci Total Environ. 729:138766. https://doi.org/10.1016/j.scitotenv.2020.138766
Malvar JL, Martín J, Orta MdM, Medina-Carrasco S, Santos JL, Aparicio I, Alonso E (2020) Simultaneous and individual adsorption of ibuprofen metabolites by a modified montmorillonite. Appl Clay Sci 189:105529. https://doi.org/10.1016/j.clay.2020.105529
Mansour F, Al-Hindi M, Yahfoufi R, Ayoub GM, Ahmad MN (2018) The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: a review. Rev Environ Sci Bio/Technol 17(1):109–145. https://doi.org/10.1007/s11157-017-9456-8
Mansouri H, Carmona RJ, Gomis-Berenguer A, Souissi-Najar S, Ouederni A, Ania CO (2015) Competitive adsorption of ibuprofen and amoxicillin mixtures from aqueous solution on activated carbons. J Colloid Interf Sci 449:252–260. https://doi.org/10.1016/j.jcis.2014.12.020
Marcelo LR, de Gois JS, da Silva AA, Cesar DV (2021) Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: a review. Environ Chem Lett 19(2):1229–1274. https://doi.org/10.1007/s10311-020-01134-2
Martín J, Orta MdM, Medina-Carrasco S, Santos JL, Aparicio I, Alonso E (2018) Removal of priority and emerging pollutants from aqueous media by adsorption onto synthetic organo-funtionalized high-charge swelling micas. Environ Res 164:488–494. https://doi.org/10.1016/j.envres.2018.03.037
Martín J, Orta MdM, Medina-Carrasco S, Santos JL, Aparicio I, Alonso E (2019) Evaluation of a modified mica and montmorillonite for the adsorption of ibuprofen from aqueous media. Appl Clay Sci 171:29–37. https://doi.org/10.1016/j.clay.2019.02.002
Mashkoor F, Nasar A, Inamuddin (2020) Carbon nanotube-based adsorbents for the removal of dyes from waters: a review. Environ Chem Lett, 18(3):605–629 https://doi.org/10.1007/s10311-020-00970-6
Matějová L, Bednárek J, Tokarský J, Koutník I, Sokolová B, Cruz GJF (2022) Adsorption of the most common non-steroidal analgesics from aquatic environment on agricultural wastes-based activated carbons; experimental adsorption study supported by molecular modeling. Appl Surf Sci 605:154607. https://doi.org/10.1016/j.apsusc.2022.154607
Mellah A, Fernandes SPS, Rodríguez R, Otero J, Paz J, Cruces J, Medina DD, Djamila H, Espiña B, Salonen LM (2018) Adsorption of pharmaceutical pollutants from water using covalent organic frameworks. Chem Eur J 24(42):10601–10605. https://doi.org/10.1002/chem.201801649
Mercurio M, Izzo F, Langella A, Grifa C, Germinario C, Daković A, Aprea P, Pasquino R, Cappelletti P, Graziano FS, Gennaro Bd (2018) Surface-modified phillipsite-rich tuff from the Campania region (southern Italy) as a promising drug carrier: an ibuprofen sodium salt trial. Am Mineralogist 103(5):700–710. https://doi.org/10.2138/am-2018-6328
Mestre AS, Pires J, Nogueira JMF, Carvalho AP (2007) Activated carbons for the adsorption of ibuprofen. Carbon 45(10):1979–1988. https://doi.org/10.1016/j.carbon.2007.06.005
Mestre AS, Pires J, Nogueira JMF, Parra JB, Carvalho AP, Ania CO (2009) Waste-derived activated carbons for removal of ibuprofen from solution: role of surface chemistry and pore structure. Bioresour Technol 100(5):1720–1726. https://doi.org/10.1016/j.biortech.2008.09.039
Mestre AS, Pires RA, Aroso I, Fernandes EM, Pinto ML, Reis RL, Andrade MA, Pires J, Silva SP, Carvalho AP (2014) Activated carbons prepared from industrial pre-treated cork: sustainable adsorbents for pharmaceutical compounds removal. Chem Eng J 253:408–417. https://doi.org/10.1016/j.cej.2014.05.051
Mestre AS, Hesse F, Freire C, Ania CO, Carvalho AP (2019) Chemically activated high grade nanoporous carbons from low density renewable biomass (Agave sisalana) for the removal of pharmaceuticals. J Colloid Interf Sci 536:681–693. https://doi.org/10.1016/j.jcis.2018.10.081
Michelon A, Bortoluz J, Raota CS, Giovanela M (2022) Agro-industrial residues as biosorbents for the removal of anti-inflammatories from aqueous matrices: an overview. Environ Adv 9:100261. https://doi.org/10.1016/j.envadv.2022.100261
Moghaddam AZ, Ghiamati E, Ayati A, Ganjali MR (2019) Application of the response surface methodology (RSM) for optimizing the adsorptive removal of chromate using a magnetic cross-linked chitosan nanocomposite. J Appl Polym Sci 136:47077. https://doi.org/10.1002/app.47077
Mohamad Nor N, Lau LC, Lee KT, Mohamed AR (2013) Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—a review. J Environ Chem Eng 1(4):658–666. https://doi.org/10.1016/j.jece.2013.09.017
Mohd Zanuri NB, Bentley MG, Caldwell GS (2017) Assessing the impact of diclofenac, ibuprofen and sildenafil citrate (Viagra®) on the fertilisation biology of broadcast spawning marine invertebrates. Marine Environ Res 127:126–136. https://doi.org/10.1016/j.marenvres.2017.04.005
Mohseni-Bandpei A, Eslami A, Kazemian H, Zarrabi M, Al-Musawi TJ (2020) A high density 3-aminopropyltriethoxysilane grafted pumice-derived silica aerogel as an efficient adsorbent for ibuprofen: characterization and optimization of the adsorption data using response surface methodology. Environ Technol Innov 18:100642. https://doi.org/10.1016/j.eti.2020.100642
Mojiri A, Andasht Kazeroon R, Gholami A (2019) Cross-linked magnetic chitosan/activated biochar for removal of emerging micropollutants from water: optimization by the artificial neural network. Water 11(3):551. https://doi.org/10.3390/w11030551
Mondal S, Aikat K, Halder G (2016) Biosorptive uptake of ibuprofen by chemically modified Parthenium hysterophorus derived biochar: equilibrium, kinetics, thermodynamics and modeling. Ecolog Eng 92:158–172. https://doi.org/10.1016/j.ecoleng.2016.03.022
Mondal S, Bobde K, Aikat K, Halder G (2016) Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J Environ Manage 182:581–594. https://doi.org/10.1016/j.jenvman.2016.08.018
Mondol MMH, Yoo DK, Jhung SH (2022) Adsorptive removal of carbamazepine and ibuprofen from aqueous solution using a defective Zr-based metal-organic framework. J Environ Chem Eng 10(6):108560. https://doi.org/10.1016/j.jece.2022.108560
Monisha RS, Mani RL, Sivaprakash B, Rajamohan N, Vo D-VN (2022) Green remediation of pharmaceutical wastes using biochar: a review. Environ Chem Lett 20(1):681–704. https://doi.org/10.1007/s10311-021-01348-y
Moreno-Pérez J, Pauletto PS, Cunha AM, Bonilla-Petriciolet Á, Salau NPG, Dotto GL (2021) Three-dimensional mass transport modeling of pharmaceuticals adsorption inside ZnAl/biochar composite. Colloid Surf A: Physicochem Eng Asp 614:126170. https://doi.org/10.1016/j.colsurfa.2021.126170
Muñiz-González A-B (2021) Ibuprofen as an emerging pollutant on non-target aquatic invertebrates: effects on Chironomus riparius. Environ Technol Innov 81:103537. https://doi.org/10.1016/j.etap.2020.103537
Naima A, Ammar F, Abdelkader O, Rachid C, Lynda H, Syafiuddin A, Boopathy R (2022) Development of a novel and efficient biochar produced from pepper stem for effective ibuprofen removal. Bioresour Technol 347:126685. https://doi.org/10.1016/j.biortech.2022.126685
Najafi M, Bastami TR, Binesh N, Ayati A, Emamverdi S (2022) Sono-sorption versus adsorption for the removal of congo red from aqueous solution using NiFeLDH/Au nanocomposite: kinetics, thermodynamics, isotherm studies, and optimization of process parameters. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2022.09.039
Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H (2006) Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res 40(17):3297–3303. https://doi.org/10.1016/j.watres.2006.06.039
Nasrollahi N, Vatanpour V, Khataee A (2022) Removal of antibiotics from wastewaters by membrane technology: limitations, successes, and future improvements. Sci Total Environ. 838:156010. https://doi.org/10.1016/j.scitotenv.2022.156010
Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS (2021) Carbon-based sustainable nanomaterials for water treatment: state-of-art and future perspectives. Chemosphere 263:128005. https://doi.org/10.1016/j.chemosphere.2020.128005
Navikaite-Snipaitiene V, Rosliuk D, Almonaityte K, Rutkaite R, Vaskeliene V, Raisutis R (2022) Ultrasound-activated modified starch microgranules for removal of ibuprofen from aqueous media. Starch - Stärke 74(5–6):2100261. https://doi.org/10.1002/star.202100261
Nawaz M, Khan AA, Hussain A, Jang J, Jung H-Y, Lee DS (2020) Reduced graphene oxide−TiO2/sodium alginate 3-dimensional structure aerogel for enhanced photocatalytic degradation of ibuprofen and sulfamethoxazole. Chemosphere 261:127702. https://doi.org/10.1016/j.chemosphere.2020.127702
Ndagijimana P, Liu X, Xu Q, Li Z, Pan B, Wang Y (2022) Simultaneous removal of ibuprofen and bisphenol A from aqueous solution by an enhanced cross-linked activated carbon and reduced graphene oxide composite. Sep Pur Technol 299:121681. https://doi.org/10.1016/j.seppur.2022.121681
Nguyen DTC, Le HTN, Do TS, Pham VT, Lam Tran D, Ho VTT, Tran TV, Nguyen DC, Nguyen TD, Bach LG, Ha HKP, Doan VT (2019) Metal-organic framework MIL-53(Fe) as an adsorbent for ibuprofen drug removal from aqueous solutions: response surface modeling and optimization. J Chem 2019:5602957. https://doi.org/10.3390/w1103055110.1155/2019/5602957
Nguyen DTC, Tran TV, Kumar PS, Din ATM, Jalil AA, Vo D-VN (2022) Invasive plants as biosorbents for environmental remediation: a review. Environ Chem Lett 20(2):1421–1451. https://doi.org/10.1007/s10311-021-01377-7
Nguyen LM, Nguyen NTT, Nguyen TTT, Nguyen TT, Nguyen DTC, Tran TV (2022) Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: a review. Environ Chem Lett 20(3):1929–1963. https://doi.org/10.1007/s10311-022-01416-x
Njaramba LK, Kim M, Yea Y, Yoon Y, Park CM (2023) Efficient adsorption of naproxen and ibuprofen by gelatin/zirconium-based metal–organic framework/sepiolite aerogels via synergistic mechanisms. Chem Eng J 452:139426. https://doi.org/10.1016/j.cej.2022.139426
Nourmoradi H, Moghadam KF, Jafari A, Kamarehie B (2018) Removal of acetaminophen and ibuprofen from aqueous solutions by activated carbon derived from Quercus Brantii (Oak) acorn as a low-cost biosorbent. J Environ Chem Eng 6(6):6807–6815. https://doi.org/10.1016/j.jece.2018.10.047
Oba, SN, Ighalo, JO, Aniagor, CO, Igwegbe, CA (2021) Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Sci Total Environ., 780:146608 doi https://doi.org/10.1016/j.scitotenv.2021.146608
Obradović M, Daković A, Smiljanić D, Ožegović M, Marković M, Rottinghaus GE, Krstić J (2022) Ibuprofen and diclofenac sodium adsorption onto functionalized minerals: equilibrium, kinetic and thermodynamic studies. Micropor Mesopor Mater 335:111795. https://doi.org/10.1016/j.micromeso.2022.111795
Ocampo-Perez R, Padilla-Ortega E, Medellin-Castillo NA, Coronado-Oyarvide P, Aguilar-Madera CG, Segovia-Sandoval SJ, Flores-Ramírez R, Parra-Marfil A (2019) Synthesis of biochar from chili seeds and its application to remove ibuprofen from water. Equilibrium and 3D modeling. Sci Total Environ 655:1397–1408. https://doi.org/10.1016/j.scitotenv.2018.11.283
Ojha A, Tiwary D, Oraon R, Singh P (2021) Degradations of endocrine-disrupting chemicals and pharmaceutical compounds in wastewater with carbon-based nanomaterials: a critical review. Environ Sci Pollut Res 28(24):30573–30594. https://doi.org/10.3390/w1103055110.1007/s11356-021-13939-x
Omorogie MO, Babalola JO, Ismaeel MO, McGettrick JD, Watson TM, Dawson DM, Carta M, Kuehnel MF (2021) Activated carbon from Nauclea diderrichii agricultural waste–a promising adsorbent for ibuprofen, methylene blue and CO2. Adv Powder Technol 32(3):866–874. https://doi.org/10.1016/j.apt.2021.01.031
Ondarts M, Reinert L, Guittonneau S, Baup S, Delpeux S, Lévêque J-M, Duclaux L (2018) Improving the adsorption kinetics of ibuprofen on an activated carbon fabric through ultrasound irradiation: simulation and experimental studies. Chem Eng J 343:163–172. https://doi.org/10.1016/j.cej.2018.02.062
Osman AI, Abd El-Monaem EM, Elgarahy AM, Aniagor CO, Hosny M, Farghali M, Rashad E, Ejimofor MI, Lopez-Maldonado EA, Ihara I, Yap PS, Rooney DW, Eltaweil AS (2023a) Methods to prepare biosorbents and magnetic sorbents for water treatment: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-023-01603-4
Osman AI, Elgarahy AM, Eltaweil AS, Abd El-Monaem EM, El-Aqapa HG, Park Y, Hwang Y, Ayati A, Farghali M, Ihara I, Al-Muhtaseb AaH, Rooney DW, Yap P-S, Sillanpää M (2023) Biofuel production, hydrogen production and water remediation by photocatalysis, biocatalysis and electrocatalysis. Environ Chem Lett. https://doi.org/10.1007/s10311-023-01581-7
Osman AI, Farghali M, Ihara I, Elgarahy AM, Ayyad A, Mehta N, Ng KH, El-Monaem EMA, Eltaweil AS, Hosny M, Hamed SM, Fawzy S, Yap PS, Rooney DW (2023c) Materials, fuels, upgrading, economy, and life cycle assessment of the pyrolysis of algal and lignocellulosic biomass: a review. Environ Chem Lett 21(3):1419–1476. https://doi.org/10.1007/s10311-023-01573-7
Oyetade OA, Martincigh BS, Skelton AA (2018) Interplay between electrostatic and hydrophobic interactions in the pH-dependent adsorption of ibuprofen onto acid-functionalized multiwalled carbon nanotubes. J Phys Chem C 122(39):22556–22568. https://doi.org/10.3390/w1103055110.1021/acs.jpcc.8b06841
Pakade VE, Tavengwa NT, Madikizela LM (2019) Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv 9(45):26142–26164. https://doi.org/10.1039/C9RA05188K
Pap S, Taggart MA, Shearer L, Li Y, Radovic S, Turk Sekulic M (2021) Removal behaviour of NSAIDs from wastewater using a P-functionalised microporous carbon. Chemosphere 264:128439. https://doi.org/10.1016/j.chemosphere.2020.128439
Park CM, Heo J, Wang D, Su C, Yoon Y (2018) Heterogeneous activation of persulfate by reduced graphene oxide–elemental silver/magnetite nanohybrids for the oxidative degradation of pharmaceuticals and endocrine disrupting compounds in water. Appl Catal B Environ 225:91–99. https://doi.org/10.1016/j.apcatb.2017.11.058
Parlak C, Alver Ö (2019) Adsorption of ibuprofen on silicon decorated fullerenes and single walled carbon nanotubes: a comparative DFT study. J. Molecul. Struct. 1184:110–113. https://doi.org/10.1016/j.molstruc.2019.02.023
Pasquino R, Di Domenico M, Izzo F, Gaudino D, Vanzanella V, Grizzuti N, de Gennaro B (2016) Rheology-sensitive response of zeolite-supported anti-inflammatory drug systems. Colloid Surf B 146:938–944. https://doi.org/10.1016/j.colsurfb.2016.07.039
Patel AK, Singhania RR, Pal A, Chen C-W, Pandey A, Dong C-D (2022) Advances on tailored biochar for bioremediation of antibiotics, pesticides and polycyclic aromatic hydrocarbon pollutants from aqueous and solid phases. Sci Total Environ 817:153054. https://doi.org/10.1016/j.scitotenv.2022.153054
Peng X, Jiang Y, Chen Z, Osman AI, Farghali M, Rooney DW, Yap P-S (2023) Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: a review. Environ Chem Lett 21(2):765–801. https://doi.org/10.1007/s10311-022-01551-5
Peralta ME, Mártire DO, Moreno MS, Parolo ME, Carlos L (2021) Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J Environ Chem Eng 9(1):104841. https://doi.org/10.1016/j.jece.2020.104841
Pereira JM, Calisto V, Santos SM (2019) Computational optimization of bioadsorbents for the removal of pharmaceuticals from water. J. Mol. Liq. 279:669–676. https://doi.org/10.1016/j.molliq.2019.01.167
Pereira AKdS, Reis DT, Barbosa KM, Scheidt GN, da Costa LS, Santos LSS (2020) Antibacterial effects and ibuprofen release potential using chitosan microspheres loaded with silver nanoparticles. Carbohyd. Polym. 488:107891. https://doi.org/10.1016/j.carres.2019.107891
Phasuphan W, Praphairaksit N, Imyim A (2019) Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. J. Mol. Liq. 294:111554. https://doi.org/10.1016/j.molliq.2019.111554
Pires BC, Dutra FVA, Borges KB (2020) Synthesis of mesoporous magnetic polypyrrole and its application in studies of removal of acidic, neutral, and basic pharmaceuticals from aqueous medium. Environ Sci Pollut Res Int 27(6):6488–6504. https://doi.org/10.1007/s11356-019-07207-2
Placente D, Benedini LA, Baldini M, Laiuppa JA, Santillán GE, Messina PV (2018) Multi-drug delivery system based on lipid membrane mimetic coated nano-hydroxyapatite formulations. Int J Pharma 548(1):559–570. https://doi.org/10.1016/j.ijpharm.2018.07.036
Prasetya N, Gede Wenten I, Franzreb M, Wöll C (2023) Metal-organic frameworks for the adsorptive removal of pharmaceutically active compounds (PhACs): Comparison to activated carbon. Coordin Chem Rev 475:214877. https://doi.org/10.1016/j.ccr.2022.214877
Priyan VV, Narayanasamy S (2022) Effective removal of pharmaceutical contaminants ibuprofen and sulfamethoxazole from water by Corn starch nanoparticles: an ecotoxicological assessment. Environ Toxicol Chem 94:103930. https://doi.org/10.1016/j.etap.2022.103930
Qamar SA, Qamar M, Basharat A, Bilal M, Cheng H, Iqbal HMN (2022) Alginate-based nano-adsorbent materials – Bioinspired solution to mitigate hazardous environmental pollutants. Chemosphere 288:132618. https://doi.org/10.1016/j.chemosphere.2021.132618
Quintelas C, Mesquita DP, Torres AM, Costa I, Ferreira EC (2020) Degradation of widespread pharmaceuticals by activated sludge: kinetic study, toxicity assessment, and comparison with adsorption processes. J Water Proc Eng 33:101061. https://doi.org/10.1016/j.jwpe.2019.1010613
Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH (2018) Removal of ibuprofen from aqueous solution by functionalized strong nano-clay composite adsorbent: kinetic and equilibrium isotherm studies. Int J Environ Sci Technol 15(3):513–524. https://doi.org/10.1007/s13762-017-1393-0
Rafati L, Ehrampoush MH, Rafati AA, Mokhtari M, Mahvi AH (2019) Fixed bed adsorption column studies and models for removal of ibuprofen from aqueous solution by strong adsorbent Nano-clay composite. J Environ Health Sci Eng 17(2):753–765. https://doi.org/10.1007/s40201-019-00392-9
Rahimzadeh G, Tajbakhsh M, Daraie M, Ayati A (2022) Heteropolyacid coupled with cyanoguanidine decorated magnetic chitosan as an efficient catalyst for the synthesis of pyranochromene derivatives. Sci Rep 12(1):17027. https://doi.org/10.1038/s41598-022-21196-2
Rainsford KD (2009) Ibuprofen: pharmacology, efficacy and safety. Inflammo Pharmacol 17:275–342
Rajab Asadi F, Hamzavi SF, Shahverdizadeh GH, Ilkhchi MG, Hosseinzadeh-Khanmiri R (2018) Dipeptide-functionalized MIL-101(Fe) as efficient material for ibuprofen delivery. Appl Organometal Chem 32(12):e4552. https://doi.org/10.1002/aoc.4552
Rameshwar SS, Sivaprakash B, Rajamohan N, Mohamed BA, Vo D-VN (2023) Remediation of tetracycline pollution using MXene and nano-zero-valent iron materials: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-023-01623-0
Ranjbari S, Tanhaei B, Ayati A, Sillanpää M (2019) Novel Aliquat-336 impregnated chitosan beads for the adsorptive removal of anionic azo dyes. Int J Biol Macromol 125:989–998. https://doi.org/10.1016/j.ijbiomac.2018.12.139
Ranjbari S, Tanhaei B, Ayati A, Khadempir S, Sillanpää M (2020) Efficient Tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: mechanism, Kinetic, Isotherms and Thermodynamic study. Int J Biol Macromol 155:421–429. https://doi.org/10.1016/j.ijbiomac.2020.03.188
Ranjbari S, Ayati A, Tanhaei B, Al-Othman A, Karimi F (2022) The surfactant-ionic liquid bi-functionalization of chitosan beads for their adsorption performance improvement toward Tartrazine. Environ Res 204:111961. https://doi.org/10.1016/j.envres.2021.111961
Reza RA, Ahmaruzzaman M (2020) A facile approach for elimination of ibuprofen from wastewater: an experimental and theoretical study. Water Environ. J 34(S1):435–443. https://doi.org/10.1111/wej.12543
Reza RA, Ahmaruzzaman M, Sil AK, Gupta VK (2014) Comparative adsorption behavior of Ibuprofen and clofibric acid onto microwave assisted activated bamboo waste. Ind Eng Chem Res 53(22):9331–9339. https://doi.org/10.1021/ie404162p
Rocha LS, Pereira D, Sousa É, Otero M, Esteves VI, Calisto V (2020) Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: a review. Sci Total Environ. 718:137272. https://doi.org/10.1016/j.scitotenv.2020.137272
Rovani S, Censi MT, Pedrotti SL, Lima ÉC, Cataluña R, Fernandes AN (2014) Development of a new adsorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. J Hazard Mater 271:311–320. https://doi.org/10.1016/j.jhazmat.2014.02.004
Sahin OI, Saygi-Yalcin B, Saloglu D (2020) Adsorption of ibuprofen from wastewater using activated carbon and graphene oxide embedded chitosan-PVA: equilibrium, kinetics, and thermodynamic and optimization with central composite design. Desal Water Treatment 179:396–417. https://doi.org/10.5004/dwt.2020.25027
Sandberg T, Weinberger C, Şen Karaman D, Rosenholm JM (2018) Modeling of a hybrid Langmuir adsorption isotherm for describing interactions between drug molecules and silica surfaces. J Pharm Sci 107(5):1392–1397. https://doi.org/10.1016/j.xphs.2017.12.025
Sandoval-González A, Robles I, Pineda-Arellano CA, Martínez-Sánchez C (2022) Removal of anti-inflammatory drugs using activated carbon from agro-industrial origin: current advances in kinetics, isotherms, and thermodynamic studies. J Iran Chem Soc 19(10):4017–4033. https://doi.org/10.1007/s13738-022-02588-7
Scheytt T, Mersmann P, Lindstädt R, Heberer T (2005) Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere 60(2):245–253. https://doi.org/10.1016/j.chemosphere.2004.12.042
Gupta SS, Bhattacharyya KG (2011) Kinetics of adsorption of metal ions on inorganic materials: a review. Adv Colloid Interface Sci 162(1):39–58. https://doi.org/10.1016/j.cis.2010.12.004
Seo PW, Bhadra BN, Ahmed I, Khan NA, Jhung SH (2016) Adsorptive removal of pharmaceuticals and personal care products from water with functionalized metal-organic frameworks: remarkable adsorbents with hydrogen-bonding abilities. Sci Rep 6(1):34462. https://doi.org/10.1038/srep34462
Shaheen JF, Sizirici B, Yildiz I (2022) Fate, transport, and risk assessment of widely prescribed pharmaceuticals in terrestrial and aquatic systems: a review. Emerg. Contam. 8:216–228. https://doi.org/10.1016/j.emcon.2022.04.001
Shahinpour A, Tanhaei B, Ayati A, Beiki H, Sillanpää M (2022) Binary dyes adsorption onto novel designed magnetic clay-biopolymer hydrogel involves characterization and adsorption performance: kinetic, equilibrium, thermodynamic, and adsorption mechanism. J Mol Liq 366:120303. https://doi.org/10.1016/j.molliq.2022.120303
Shen T, Han T, Zhao Q, Ding F, Mao S, Gao M (2022) Efficient removal of mefenamic acid and ibuprofen on organo-Vts with a quinoline-containing gemini surfactant: adsorption studies and model calculations. Chemosphere 295:133846. https://doi.org/10.1016/j.chemosphere.2022.133846
Shin J, Lee Y-G, Lee S-H, Kim S, Ochir D, Park Y, Kim J, Chon K (2020) Single and competitive adsorptions of micropollutants using pristine and alkali-modified biochars from spent coffee grounds. J Hazard Mater 400:123102. https://doi.org/10.1016/j.jhazmat.2020.123102
Shin J, Kwak J, Lee Y-G, Kim S, Choi M, Bae S, Lee S-H, Park Y, Chon K (2021) Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: contribution of hydrophobic and π–π interactions. Environ Pollut 270:116244. https://doi.org/10.1016/j.envpol.2020.116244
Shin J, Kwak J, Kim S, Son C, Lee Y-G, Baek S, Park Y, Chae K-J, Yang E, Chon K (2022) Facilitated physisorption of ibuprofen on waste coffee residue biochars through simultaneous magnetization and activation in groundwater and lake water: adsorption mechanisms and reusability. J Environ Chem Eng 10(3):107914. https://doi.org/10.1016/j.jece.2022.107914
Show S, Mukherjee S, Devi MS, Karmakar B, Halder G (2021) Linear and non-linear analysis of Ibuprofen riddance efficacy by Terminalia catappa active biochar: equilibrium, kinetics, safe disposal, reusability and cost estimation. Proc Safe Environ Protect 147:942–964. https://doi.org/10.1016/j.psep.2021.01.024
Show S, Karmakar B, Halder G (2022) Sorptive uptake of anti-inflammatory drug ibuprofen by waste biomass–derived biochar: experimental and statistical analysis. Biomass Conver Bioref 12(9):3955–3973. https://doi.org/10.1007/s13399-020-00922-8
Show S, Sarkhel R, Halder G (2022) Elucidating sorptive eradication of ibuprofen using calcium chloride caged bentonite clay and acid activated alginate beads in a fixed bed upward flow column reactor. Sustain Chem Pharm 27:100698. https://doi.org/10.1016/j.scp.2022.100698
Silva SP, Sabino MA, Fernandes EM, Correlo VM, Boesel LF, Reis RL (2005) Cork: properties, capabilities and applications. Int Mater Rev 50(6):345–365. https://doi.org/10.1179/174328005X41168
Silva A, Coimbra RN, Escapa C, Figueiredo SA, Freitas OM, Otero M (2020) Green microalgae scenedesmus obliquus utilization for the adsorptive removal of nonsteroidal anti-inflammatory drugs (NSAIDs) from Water Samples. Int J Environ Res Public Health, 17(10):3707 https://www.mdpi.com/1660-4601/17/10/3707
Singh S, Kumar V, Datta S, Dhanjal DS, Sharma K, Samuel J, Singh J (2020) Current advancement and future prospect of biosorbents for bioremediation. Sci Total Environ 709:135895. https://doi.org/10.1016/j.scitotenv.2019.135895
Sivarajasekar N, Mohanraj N, Sivamani S, Prakash Maran J, Ganesh Moorthy I, Balasubramani K (2018) Statistical optimization studies on adsorption of ibuprofen onto Albizialebbeck seed pods activated carbon prepared using microwave irradiation. Mater Today Proc 5(2):7264–7274. https://doi.org/10.1016/j.matpr.2017.11.394
Skwierawska A, Nowacka D, Kozłowska-Tylingo K (2022) Removal of nonsteroidal anti-inflammatory drugs and analgesics from wastewater by adsorption on cross-linked β-cyclodextrin. Water Res Ind 28:100186. https://doi.org/10.1016/j.wri.2022.100186
Smiljanić D, de Gennaro B, Daković A, Galzerano B, Germinario C, Izzo F, Rottinghaus GE, Langella A (2021) Removal of non-steroidal anti-inflammatory drugs from water by zeolite-rich composites: the interference of inorganic anions on the ibuprofen and naproxen adsorption. J Environ Manage 286:112168. https://doi.org/10.1016/j.jenvman.2021.112168
Sompornpailin D, Mongconpattarasuk P, Ratanatawanate C, Apiratikul R, Chu KH, Punyapalakul P (2022) Adsorption of nonsteroidal anti-inflammatory drugs onto composite beads of a 1D flexible framework MIL-53(Al): adsorption mechanisms and fixed-bed study. J Environ Chem Eng 10(4):108144. https://doi.org/10.1016/j.jece.2022.108144
Sophia A, Lima EC (2018) Removal of emerging contaminants from the environment by adsorption. Ecotoxic Environ Saf 150:1–17. https://doi.org/10.1016/j.ecoenv.2017.12.026
Souza MPCd, Sábio RM, Ribeiro TdC, Santos AMd, Meneguin AB, Chorilli M (2020) Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int J Biol Macromol 159:804–822. https://doi.org/10.1016/j.ijbiomac.2020.05.104
Sruthi L, Janani B, Sudheer Khan S (2021) Ibuprofen removal from aqueous solution via light-harvesting photocatalysis by nano-heterojunctions: a review. Sep Pur Technol 279:119709. https://doi.org/10.1016/j.seppur.2021.119709
Streit AFM, Collazzo GC, Druzian SP, Verdi RS, Foletto EL, Oliveira LFS, Dotto GL (2021) Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 262:128322. https://doi.org/10.1016/j.chemosphere.2020.128322
Sun W, Li H, Li H, Li S, Cao X (2019) Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: batch experiment and DFT calculation. Chem Eng J 360:645–653. https://doi.org/10.1016/j.cej.2018.12.021
Suttiruengwong S, Pivsa-Art S, Chareonpanich M (2018) Hydrophilic and hydrophobic mesoporous silica derived from rice husk ash as a potential drug carrier. Mater (Basel). https://doi.org/10.3390/ma11071142
Tabatabaeian K, Simayee M, Fallah Shojaie A, Mashayekhi F, Hadavi M (2020) The effect of silica coating on the drug release profile and biocompatibility of nano-MOF-5. J Sci Islam Rep Iran 31(1):51–62. https://doi.org/10.22059/jsciences.2020.285236.1007428
Tabrizi SH, Tanhaei B, Ayati A, Ranjbari S (2022) Substantial improvement in the adsorption behavior of montmorillonite toward Tartrazine through hexadecylamine impregnation. Environ Res 204(Part A):111965. https://doi.org/10.1016/j.envres.2021.111965
Taheran M, Brar SK, Verma M, Surampalli RY, Zhang TC, Valero JR (2016) Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci Total Environ 547:60–77. https://doi.org/10.1016/j.scitotenv.2015.12.139
Takam B, Tarkwa J-B, Acayanka E, Nzali S, Chesseu DM, Kamgang GY, Laminsi S (2020) Insight into the removal process mechanism of pharmaceutical compounds and dyes on plasma-modified biomass: the key role of adsorbate specificity. Environ Sci Pollut Res 27(16):20500–20515. https://doi.org/10.1007/s11356-020-08536-3
Tan D, Yuan P, Annabi-Bergaya F, Yu H, Liu D, Liu H, He H (2013) Natural halloysite nanotubes as mesoporous carriers for the loading of ibuprofen. Micropor Mesopor Mater 179:89–98. https://doi.org/10.1016/j.micromeso.2013.05.007
Tanhaei B, Ayati A, Lahtinen M, Sillanpää M (2015) Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of methyl orange adsorption. Chem Eng J 259:1–10. https://doi.org/10.1016/j.cej.2014.07.109
Tanhaei B, Ayati A, Sillanpää M (2019) Magnetic xanthate modified chitosan as an emerging adsorbent for cationic azo dyes removal: kinetic, thermodynamic and isothermal studies. Int J Biol Macromol 121:1126–1134. https://doi.org/10.1016/j.ijbiomac.2018.10.137
Tanhaei B, Ayati A, Iakovleva E, Sillanpää M (2020) Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: synthesis, characterization and adsorption study. Int J Biol Macromol 164:3621–3631. https://doi.org/10.1016/j.ijbiomac.2020.08.207
Taoufik N, Boumya W, Janani FZ, Elhalil A, Mahjoubi FZ, barka, N, (2020) Removal of emerging pharmaceutical pollutants: a systematic map** study review. J Environ Chem Eng 8(5):104251. https://doi.org/10.1016/j.jece.2020.104251
Tee GT, Gok XY, Yong WF (2022) Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: a review. Environ Res 212:113248. https://doi.org/10.1016/j.envres.2022.113248
Thakur K, Kandasubramanian B (2019) Graphene and graphene oxide-based composites for removal of organic pollutants: a review. J Chem Eng Data 64(3):833–867. https://doi.org/10.1021/acs.jced.8b01057
Tian W, Lin J, Zhang H, Duan X, Wang H, Sun H, Wang S (2022) Kinetics and mechanism of synergistic adsorption and persulfate activation by N-doped porous carbon for antibiotics removals in single and binary solutions. J Hazard Mater 423:127083. https://doi.org/10.1016/j.jhazmat.2021.127083
Titchou FE, Zazou H, Afanga H, El Gaayda J, Akbour RA, Hamdani M (2021) Removal of persistent organic pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process. Groundw Sustain Dev 13:100575. https://doi.org/10.1016/j.gsd.2021.100575
Tiwari B, Sellamuthu B, Ouarda Y, Drogui P, Tyagi RD, Buelna G (2017) Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour Technol 224:1–12. https://doi.org/10.1016/j.biortech.2016.11.042
Torrellas SÁ, García Lovera R, Escalona N, Sepúlveda C, Sotelo JL, García J (2015) Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem Eng J 279:788–798. https://doi.org/10.1016/j.cej.2015.05.104
Trzeciak K, Kaźmierski S, Wielgus E, Potrzebowski MJ (2020) DiSupLo - New extremely easy and efficient method for loading of active pharmaceutical ingredients into the pores of MCM-41 mesoporous silica particles. Micropor Mesopor Mater 308:110506. https://doi.org/10.1016/j.micromeso.2020.110506
Tseng LY, You C, Vu C, Chistolini MK, Wang CY, Mast K, Luo F, Asvapathanagul P, Gedalanga PB, Eusebi AL, Gorbi S, Pittura L, Fatone F (2022) Adsorption of contaminants of emerging concern (CECs) with varying hydrophobicity on macro- and microplastic polyvinyl chloride, polyethylene, and polystyrene: kinetics and potential mechanisms. Water 14(16):2581. https://doi.org/10.3390/w14162581
Turk Sekulic M, Boskovic N, Slavkovic A, Garunovic J, Kolakovic S, Pap S (2019) Surface functionalised adsorbent for emerging pharmaceutical removal: adsorption performance and mechanisms. Proc Safe Environ Protect 125:50–63. https://doi.org/10.1016/j.psep.2019.03.007
Ulfa M, Iswanti Y (2020) Ibuprofen adsorption study by langmuir, freundlich, temkin and dubinin-radushkevich models using nano zinc oxide from mild hydrothermal condition. IOP Conf Series: Mater Sci Eng. 833(1):012096. https://doi.org/10.1088/1757-899X/833/1/012096
Ulfa M, Aristia KS, Prasetyoko D (2018) Synthesis of mesoporous silica materials via dual templating method from starch of waste rice and their application for drug delivery system. AIP Conference Proceed 2049(1):020002. https://doi.org/10.1063/1.5082407
Ulfa M, Sari AY, Prasetyoko D (2018) Synthesis of unique natural silica (UNS) material via dual co-templating method using starch of waste rice-gelatin composite and their performance in drug delivery system. AIP Conference Proceed 2049(1):020003. https://doi.org/10.1063/1.5082408
Ulfa M, Saraswati TE, Mulyani B (2019) ) Adsorption of ibuprofen molecule onto mesoporous silica SBA-15 loaded by iron particles using arc discharge treatment. IOP Conf Ser: Mater Sci Eng 509:012073. https://doi.org/10.1088/1757-899X/509/1/012073
Ulfa M, Worabay NS, Pradipta MF, Prasetyoko D (2019) Removal of ibuprofen from aqueous solutions by adsorption on tiny zinc oxide sheet-like structure. AIP Conf Proc 2194(1):020131. https://doi.org/10.1063/1.5139863
Ulfa M, Fadhila YLE, Prasetyoko D (2020) Activation of carbon at different concentration microsphere adsorbent and its application for ibuprofen adsorption. J Phys: Conf Ser 1567(4):042008. https://doi.org/10.1088/1742-6596/1567/4/042008
Ulfa M, Gumilar IM, Prasetyoko D (2020) Alkaline activation of marble-like carbon structure and it’s application for inflammatory adsorption. J Phys: Conf Ser 1503:012028. https://doi.org/10.1088/1742-6596/1503/1/012028
Van Limbergen T, Roegiers IH, Bonné R, Mare F, Haeldermans T, Joos B, Nouwen O, Manca JV, Vangronsveld J, Thijs S (2022) Characterisation of two wood-waste and coffee bean husk biochars for the removal of micropollutants from water. Front Environ Sci. https://doi.org/10.3389/fenvs.2022.814267
Van Tran T, Cam Nguyen DT, Le HTN, Nguyen OTK, Nguyen VH, Nguyen TT, Bach LG, Nguyen TD (2019) A hollow mesoporous carbon from metal-organic framework for robust adsorbability of ibuprofen drug in water Royal Soc. Open Sci 6(5):190058. https://doi.org/10.1098/rsos.190058
Van Tran T, Nguyen DTC, Le HTN, Bach LG, Vo D-VN, Dao T-UT, Lim KT, Nguyen TD (2019) Effect of thermolysis condition on characteristics and nonsteroidal anti-inflammatory drugs (NSAIDs) absorbability of Fe-MIL-88B-derived mesoporous carbons. J Environ Chem Eng 7(5):103356. https://doi.org/10.1016/j.jece.2019.103356
Varghese RT, Cherian RM, Antony T, Tharayil A, Das H, Kargarzadeh H, Chirayil CJ, Thomas S (2022) A review on the best bioadsorbent membrane- nanocellulose for effective removal of pollutants from aqueous solutions. Carbohydr Polym Technol Appl 3:100209. https://doi.org/10.1016/j.carpta.2022.100209
Vargues F, Brion MA, Rosa da Costa AM, Moreira JA, Ribau Teixeira M (2021) Development of a magnetic activated carbon adsorbent for the removal of common pharmaceuticals in wastewater treatment. Int J Environ Sci Technol 18(9):2805–2818. https://doi.org/10.1007/s13762-020-03029-9
Vicente-Martínez Y, Caravaca M, Soto-Meca A, Solana-González R (2020) Magnetic core-modified silver nanoparticles for ibuprofen removal: an emerging pollutant in waters. Sci Rep 10(1):18288. https://doi.org/10.1038/s41598-020-75223-1
Vieno NM, Tuhkanen T, Kronberg L (2005) Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ Sci Technol 39(21):8220–8226. https://doi.org/10.1021/es051124k
Villabona-Ortíza Á, Tejada-Tovara C, Ortega-Torob R (2021) Kinetics and adsorption isotherms of the removal of ibuprofen on a porous adsorbent made from agroindustrial waste. Desal Water Treatment 209:316–323. https://doi.org/10.5004/dwt.2021.26538
Wang J, Yang F (2021) Preparation of 2-hydroxypropyl-β-cyclodextrin polymers crosslinked by poly(acrylic acid) for efficient removal of ibuprofen. Mater. Lett. 284:128882. https://doi.org/10.1016/j.matlet.2020.128882
Wang Z, Huang Q, Yu Y, Wang C, Ou W, Peng X (2013) Stereoisomeric profiling of pharmaceuticals ibuprofen and iopromide in wastewater and river water China. Environ Geochem Health 35(5):683–691. https://doi.org/10.1007/s10653-013-9551-x
Wang Q, ** X, Bai S, Sun J (2017) Polyacrylic acid (PAA)- surface grafted dense nanosilica spheres for ibuprofen delivery. J Control Release 259:e107–e108. https://doi.org/10.1016/j.jconrel.2017.03.226
Wang X, Zhuo N, Fu C, Tian Z, Li H, Zhang J, Wu W, Yang Z, Yang W (2017) Enhanced selective adsorption of benzotriazole onto nanosized zeolitic imidazolate frameworks confined in polystyrene anion exchanger. Chem Eng J 328:816–824. https://doi.org/10.1016/j.cej.2017.07.095
Wang X, Yin R, Zeng L, Zhu M (2019) A review of graphene-based nanomaterials for removal of antibiotics from aqueous environments. Environ Pollut 253:100–110. https://doi.org/10.1016/j.envpol.2019.06.067
Wang Y, Li W, Liu T, Xu L, Guo Y, Ke J, Li S, Li H (2019) Design and preparation of mesoporous silica carriers with chiral structures for drug release differentiation. Mater Sci Eng C 103:109737. https://doi.org/10.1016/j.msec.2019.109737
Wang D, Chen X, Feng J, Sun M (2022) Recent advances of ordered mesoporous silica materials for solid-phase extraction. J Chromatogr A 1675:463157. https://doi.org/10.1016/j.chroma.2022.463157
Wasilewska M, Deryło-Marczewska A (2022) Adsorption of non-steroidal anti-inflammatory drugs on alginate-carbon composites-equilibrium and kinetics. Materials 15(17):6049. https://doi.org/10.3390/ma15176049
Wazzan N (2021) Adsorption of non-steroidal anti-inflammatory drugs (NSAIDs) on nanographene surface: density functional theory study. Arab J Chem 14(3):103002. https://doi.org/10.1016/j.arabjc.2021.103002
Wei J, Zhang W, Pan W, Li C, Sun W (2018) Experimental and theoretical investigations on Se(iv) and Se(vi) adsorption to UiO-66-based metal–organic frameworks. Environ Sci Nano 5(6):1441–1453. https://doi.org/10.1039/C8EN00180D
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Clean Prod 175:361–375. https://doi.org/10.1016/j.jclepro.2017.12.059
Wu C, Witter JD, Spongberg AL, Czajkowski KP (2009) Occurrence of selected pharmaceuticals in an agricultural landscape, western Lake Erie basin. Water Res 43(14):3407–3416. https://doi.org/10.1016/j.watres.2009.05.014
Wu T, Prasetya N, Li K (2022) Re-generable and re-synthesisable micro-structured MIL-53 Rachig Rings for ibuprofen removal. J Environ Chem Eng 10(3):107432. https://doi.org/10.1016/j.jece.2022.107432
**ong P, Zhang H, Li G, Liao C, Jiang G (2021) Adsorption removal of ibuprofen and naproxen from aqueous solution with Cu-doped Mil-101(Fe). Sci Total Environ 797:149179. https://doi.org/10.1016/j.scitotenv.2021.149179
Xu T, Hou X, Liu S, Liu B (2018) One-step synthesis of magnetic and porous Ni@MOF-74(Ni) composite. Micropor Mesopor Mater 259:178–183. https://doi.org/10.1016/j.micromeso.2017.10.014
Yan M, Wu Y (2020) Fabrication and evaluation of bioinspired pDA@TiO2-based ibuprofen-imprinted nanocomposite membranes for highly selective adsorption and separation applications. New J Chem 44(25):10703–10712. https://doi.org/10.1039/D0NJ01836H
Yang X, Wang J, El-Sherbeeny AM, AlHammadi AA, Park W-H, Abukhadra MR (2022) Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: steric, energetic, and oxidation studies. Chem Eng J 431:134312. https://doi.org/10.1016/j.cej.2021.134312
Yazidi A, Sellaoui L, Dotto GL, Bonilla-Petriciolet A, Fröhlich AC, Lamine AB (2019) Monolayer and multilayer adsorption of pharmaceuticals on activated carbon: application of advanced statistical physics models. J Mol Liq 283:276–286. https://doi.org/10.1016/j.molliq.2019.03.101
Yin R, Sun J, **ang Y, Shang C (2018) Recycling and reuse of rusted iron particles containing core-shell Fe-FeOOH for ibuprofen removal: Adsorption and persulfate-based advanced oxidation. J Clean Prod 178:441–448. https://doi.org/10.1016/j.jclepro.2018.01.005
Yu F, Bai X, Liang M, Ma J (2021) Recent progress on metal-organic framework-derived porous carbon and its composite for pollutant adsorption from liquid phase. Chem Eng J 405:126960. https://doi.org/10.1016/j.cej.2020.126960
Yu M, Li H, **e J, Xu Y, Lu X (2022) A descriptive and comparative analysis on the adsorption of PPCPs by molecularly imprinted polymers. Talanta 236:122875. https://doi.org/10.1016/j.talanta.2021.122875
Yudha SP, Tekasakul S, Phoungthong K, Chuenchom L (2019) Green synthesis of low-cost and eco-friendly adsorbent for dye and pharmaceutical adsorption: kinetic, isotherm, thermodynamic and regeneration studies. Mater Res Express 6(12):125526. https://doi.org/10.1088/2053-1591/ab58ae
Zare EN, Fallah Z, Le VT, Doan V-D, Mudhoo A, Joo S-W, Vasseghian Y, Tajbakhsh M, Moradi O, Sillanpää M, Varma RS (2022) Remediation of pharmaceuticals from contaminated water by molecularly imprinted polymers: a review. Environ Chem Lett 20(4):2629–2664. https://doi.org/10.1007/s10311-022-01439-4
Zeng H, Gao M, Shen T, Ding F (2018) Organo silica nanosheets with gemini surfactants for rapid adsorption of ibuprofen from aqueous solutions. J Taiwan Inst Chem Eng 93:329–335. https://doi.org/10.1016/j.jtice.2018.07.038
Zhang S, Zhang Q, Darisaw S, Ehie O, Wang G (2007) Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA. Chemosphere 66(6):1057–1069. https://doi.org/10.1016/j.chemosphere.2006.06.067
Zhang W, Gago-Ferrero P, Gao Q, Ahrens L, Blum K, Rostvall A, Björlenius B, Andersson PL, Wiberg K, Haglund P, Renman G (2019) Evaluation of five filter media in column experiment on the removal of selected organic micropollutants and phosphorus from household wastewater. J. Environ. Manag. 246:920–928. https://doi.org/10.1016/j.jenvman.2019.05.137
Zhang G, Li S, Shuang C, Mu Y, Li A, Tan L (2020) The effect of incorporating inorganic materials into quaternized polyacrylic polymer on its mechanical strength and adsorption behaviour for ibuprofen removal. Sci Rep 10(1):5188. https://doi.org/10.1038/s41598-020-62153-1
Zhao H, Liu X, Cao Z, Zhan Y, Shi X, Yang Y, Zhou J, Xu J (2016) Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J Hazard Mater 310:235–245. https://doi.org/10.1016/j.jhazmat.2016.02.045
Zhao Y, Choi J-W, Lin S, Kim J-A, Cho C-W, Yun Y-S (2018) Experimental and QSAR studies on adsorptive interaction of anionic nonsteroidal anti-inflammatory drugs with activated charcoal. Chemosphere 212:620–628. https://doi.org/10.1016/j.chemosphere.2018.08.115
Zhao R, Ma T, Li S, Tian Y, Zhu G (2019) Porous aromatic framework modified electrospun fiber membrane as a highly efficient and reusable adsorbent for pharmaceuticals and personal care products removal. ACS Appl Mater Interfaces 11(18):16662–16673. https://doi.org/10.1021/acsami.9b04326
Zheng Y, Cheng B, Fan J, Yu J, Ho W (2021) Review on nickel-based adsorption materials for Congo red. J Hazard Mater 403:123559. https://doi.org/10.1016/j.jhazmat.2020.123559
Żółtowska-Aksamitowska S, Bartczak P, Zembrzuska J, Jesionowski T (2018) Removal of hazardous non-steroidal anti-inflammatory drugs from aqueous solutions by biosorbent based on chitin and lignin. Sci Total Environ 612:1223–1233. https://doi.org/10.1016/j.scitotenv.2017.09.037
Acknowledgements
This work was financially supported by the Government of the Russian Federation through the ITMO Fellowship and Professorship Program. Dr Ahmed I. Osman and Prof. David W. Rooney wish to acknowledge the support of The Bryden Centre project (Project ID VA5048), which was awarded by The European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB), with match funding provided by the Department for the Economy in Northern Ireland and the Department of Business, Enterprise and Innovation in the Republic of Ireland.
Funding
This work was financially supported by the Government of the Russian Federation through the ITMO Fellowship. The article was funded by SEUPB, Bryden Centre project (Project ID VA5048).
Author information
Authors and Affiliations
Contributions
AIO Conceptualization, Review, Review & editing. AA Data gathering, Methodology, Writing & Review. MF Conceptualization, Review, Review & editing. PK Writing & Review. BT Data gathering, Writing & Review. EK Writing & Review. PT Data gathering. CT Review & editing. HKM Writing & Review. AA: Writing & Review. II Writing & Review. DWR Writing & Review. MS: Writing & Review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The views and opinions expressed in this review do not necessarily reflect those of the European Commission or the Special EU Programmes Body (SEUPB).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osman, A.I., Ayati, A., Farghali, M. et al. Advanced adsorbents for ibuprofen removal from aquatic environments: a review. Environ Chem Lett 22, 373–418 (2024). https://doi.org/10.1007/s10311-023-01647-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01647-6