Abstract
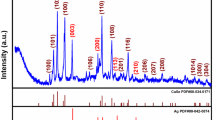
Bimetallic nanoparticles (BMNPs) have received considerable attention due to their distinctive properties when compared to the corresponding monometallic NPs and their bulk counterpart. In this report, the formation of gold@silver core-shell nanoparticles (Au@AgCSNPs) was achieved via a one-pot synthetic approach after mixing 1:1 M solutions of Au and Ag ions. L-cysteine was used as reducing as well as cap** agent for preparing Au@AgCSNPs. Ultraviolet-visible (UV-Vis) spectroscopy was employed for surface plasmon study while Fourier-transform infrared (FTIR) spectroscopy gave insights for interaction of NPs with specific functionality of the cap** material. Surface morphology of the fabricated Au@AgCSNPs, probed by atomic force microscopy (AFM), indicated an average height of nanoparticles around 43 ± 3 nm and their crystallinity were confirmed via powder X-ray diffraction (PXRD) study. Significantly, the as synthesized Au@AgCSBMNPs were fabricated onto the conductive surface of glassy carbon electrode (GCE), stabilized with nafion, and then utilized as an extremely sensitive/greatly selective sensor for voltammetric detection of Hg2+. The developed sensor responded linearly to Hg2+ between 0.001 and 19 ppb with limit of detection (LOD) as low as 0.0001 ppb (0.1 ppt). Finally, the sensor was effectively applied for Hg2+ detection in different groundwater samples and is workable at concentrations undetectable by several sensing tools.

.




Similar content being viewed by others
References
Solano-Umaña V, Vega-Baudrit JR, González-Paz R (2015) The new field of the nanomedicine. Int J Appl Sci Technol 5:79–88
Ghosh SK, Mandal M, Kundo S, Nath S, Pal T (2004) Bimetallic Pt–Ni nanoparticles can catalyze reduction of aromatic nitro compounds by sodium borohydride in aqueous solution. Appl Catal A Gen 268(1–2):61–66
Shin Y, Bae IT, Arey BW, Exarhos GJ (2013) Facile stabilization of gold-silver alloy nanoparticles on cellulose nanocrystal. Langmuir 29:4901–4907
Moghimi N, Mohapatra M, Leung KT (2015) Bimetallic nanoparticles for arsenic detection. Anal Chem 87(11):5546–5552
An Y, Li T, ** Z, Dong M, **a H, Wang X (2010) Effect of bimetallic and polymer-coated Fe nanoparticles on biological denitrification. Bioresour Technol 101(24):9825–9828
Zaleska-Medynska A, Marchelek M, Diak M, Grabowska E (2016) Noble metal-based bimetallic nanoparticles: the effect of the structure on the optical, catalytic and photocatalytic properties. Adv Colloid Interf Sci 229:80–107
Wen J, Tian Y (2016) The synthesis of Cu-Ag core-shell bimetallic nanoparticles for IC bonding, 17th International Conference on Electronic Packaging Technology, 16–19 August, Wuhan, China
Boote BW, Byun H, Kim JH (2014) Silver-gold bimetallic nanoparticles and their applications as optical materials. J Nanosci Nanotechnol 14(2):1563–1577
Reza M, Hosseini M, Jamalabadi H, Najafi M (2013) Bimetallic nanoparticles as a novel chemiresistor coating. J Iran Chem Soc 10:783–789
Zhang C, Chen BQ, Li ZY (2015) Surface plasmon resonance in bimetallic core−shell nanoparticles. Phys Chem C 119(29):16836–16845
Iswarya CN, Daniel SCGK, Sivakumar M (2017) Studies on l-histidine capped silver and gold nanoparticle for dopamine detection. Mater Sci Eng C 75:393–401
Selvakannan PR, Swami A, Srisathiyanarayanan D, Shirude PS, Pasricha R, Mandale AB, Sastry M (2004) Synthesis of aqueous Au core-Ag shell nanoparticles using tyrosine as a pH-dependent reducing agent and assembling phase-transferred silver nanoparticles at the air-water interface. Langmuir 20(18):7825–7836
Sharma B, Rabina MK (2015) Biologically active l-cysteine as a reducing/cap** agent for controlled tuning of gold nanoparticles. J Alloys Compd 649:11–18
Chai F, Wang C, Wang T, Ma Z, Su Z (2010) L-cysteine functionalized gold nanoparticles for the colorimetric detection of Hg2+ induced by ultraviolet light. Nanotechnology 21(2):025501
Sirajuddin NA, Afridi HI, Hassan SS, Shah A, Niaz A (2011) Direct synthesis and stabilization of bi-sized cysteine-derived gold nanoparticles: reduction catalyst for methylene blue. J Iran Chem Soc 8(S1):S34–S43
Aryal S, Dharmaraj BKCRN, Bhattarai N, Kim CH, Ki HY (2006) Spectroscopic identification of S-Au interaction in cysteine capped gold nanoparticles. Spectrochim Acta A 63(1):160–163
Wang WX (2012) Biodynamic understanding of mercury accumulation in marine and freshwater fish. Adv Environ Res 1(1):15–35
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Pub Health Article ID 460508:1–10
Bruno CJ, Luiz CSFF, Luiz HMJ, Silvéria PNS, Edenir RPF, Orlando FF (2011) Development of carbon nanotubes paste electrode modified with cross-linked chitosan for cadmium (II) and mercury (II) determination. J Electroanal Chem 660:209–216
Memon AG, Zhou X, Liu J, Wang R, Liu L, Yu B, He M, Shi H (2017) Utilization of unmodified gold nanoparticles for label-free detection of mercury (II): insight into rational design of mercury-specific oligonucleotides. J Hazard Mater 321:417–423
Lokhande AC, Shinde NM, Shelke A, Babar PT, Kim JH (2017) Reliable and reproducible colorimetric detection of mercury ions (Hg2+) using green synthesized optically active silver nanoparticles containing thin film on flexible plastic substrate. J Solid State Electrochem 21(9):2747–2751
Silva MF, Tóth IV, Range AO (2006) Determination of mercury in fish by cold vapor atomic absorption spectrophotometry using a multicommuted flow injection analysis system. Anal Sci 22(6):861–864
Ebdon L, Foulkes MES, Roux SL, Muñoz-Olivas R (2002) Cold vapor atomic fluorescence spectrometry and gas chromatography-pyrolysis-atomic fluorescence spectrometry for routine determination of total and organometallic mercury in food samples. Analyst 127(8):1108–1114
Passariello B, Barbaro M, Quaresima S, Casciello A, Marabini A (1996) Determination of mercury by inductively coupled plasma—mass spectrometry. Microchem J 54(4):348–354
Zhu X, Alexandratos SD (2007) Determination of trace levels of mercury in aqueous solutions by inductively coupled plasma atomic emission spectrometry: elimination of the memory effect. Microchem J 86(1):37–41
Gao C, Huang XJ (2013) Voltammetric determination of mercury (II). Trends Anal Chem 51:1–12
Walcarius A, Devoy J, Bessiere J (2000) Silica-modified electrode for the selective detection of mercury. J Solid State Electrochem 4(6):330–336
Shahar T, Tal N, Mandler D (2013) The synthesis and characterization of thiol-based aryl diazonium modified glassy carbon electrode for the voltammetric determination of low levels of Hg(II). J Solid State Electrochem 17(6):1543–1552
Yu J, Guan H, Chi D (2017) An amperometric glucose oxidase biosensor based on liposome microreactor-chitosan nanocomposite-modified electrode for determination of trace mercury. J Solid State Electrochem 21(4):1175–1183
Gayathri J, Selvan KS, Narayanan SS (2018) A novel sensor for the determination of Hg2+ in waters based on octadentate ligand immobilized multi-walled carbon nanotube attached to paraffin wax impregnated graphite electrodes (PIGE). 22:2879–2888
Kassab LRP, de Araújo CB, Kobayashi RA, De RD, Pinto A, da Silva DM (2007) Influence of silver nanoparticles in the luminescence efficiency of Pr3+-doped tellurite glasses. J Appl Phys 102(10):103515
Kassab LRP, Bomfim FA, Martinelli JR (2009) Energy transfer and frequency up conversion in Yb3+–Er3+-doped PbO-GeO2 glass containing silver nanoparticles. J Appl Phys B94:239–242
Ghadimi A, Metselaar HSC, Lotfizadehdehkordi B (2015) Nanofluid stability optimization based on UV-Vis spectrophotometer measurement. J Engineer Sci Tech Special Issue on SOMCHE 2014 & RSCE 2014 Conference, January 32 – 40
Kirubha E, Palanisamy PK (2014) Green synthesis, characterization of Au–Ag core–shell nanoparticles using gripe water and their applications in nonlinear optics and surface enhanced Raman studies. Adv Nat Sci Nanosci Nanotechnol 5:1–6
Mandal S, Gole A, Lala N, Gonnade R, Ganvir V, Sastry M (2001) Studies on the reversible aggregation of cysteine-capped colloidal silver particles interconnected via hydrogen bonds. Langmuir 17(20):6262–6268
Nagaonkar D, Rai M (2015) Sequentially reduced biogenic silver-gold nanoparticles with enhanced antimicrobial potential over silver and gold monometallic nanoparticles. Adv Mater Lett 6(4):334–341
Li N, Wang C, Li T, Latimer B, Liu Z, Tang Z (2018) Au@Ag core-shell nanoparticles supported on carbon nanotubes as promising catalysts for oxygen electroreduction. Int J Electrochem Sci 13:6756–6770
Hassan SS, Nafady A, Sirajuddin SAR, Kalhoro MS, Abro MI, Sherazi STH (2015) Ultra-trace level electrochemical sensor for methylene blue dye based on nafion stabilized ibuprofen derived gold nanoparticles. Sensors Actuators B Chem 208:320–326
Tagar ZA, Sirajuddin MN, Agheem MH, Junejo Y, Hassan SS, Kalwar NH, Khattak MI (2011) Selective, simple and economical lead sensor based on ibuprofen derived silver nanoparticles. Sensors Actuators B Chem 157(2):430–437
Liao Y, Li Q, Yue Y, Shao S (2015) Selective electrochemical determination of trace level copper using a salicylaldehyde azine/MWCNTs/nafion modified pyrolytic graphite electrode by the anodic strip** voltammetric method. RSC Adv 5(5):3232–3238
Hassan SS, Sirajuddin, Solangi AR, Kazi TG, Kalhoro MS, Junejo Y, Tagar ZA, Kalwar NH (2012) Nafion stabilized ibuprofen–gold nanostructures modified screen printed electrode as arsenic(III) sensor. J Electroanal Chem 682:77–82
Shaikh T, Nafady A, Talpur FN, Sirajuddin AMH, Shah MR, Sherazi STH, Soomro RA, Siddiqui S (2015) Tranexamic acid derived gold nanoparticles modified glassy carbon electrode as sensitive sensor for determination of nalbuphine. Sensors Actuators B Chem 211:359–369
Ugo P, Cavalieri F, Rudello D, Moretto L, Argese E (2001) Nafion coated electrodes as voltammetric sensors for iron analysis in sediments and pore waters: an example from the Lagoon of Venice. Sensors 1(4):102–113
Rahman NA, Yusof NA, Maamor NAM, Noor SMM (2017) Development of electrochemical sensor for simultaneous determination of Cd(II) and Hg(II) ion by exploiting newly synthesized cyclic dipeptide. Int J Electrochem Sci 7:186–196
**ong E, Zhang X, Liu Y, Zhou J, Yu P, Chen J (2016) An electrochemical biosensor for sensitive detection of Hg2+ based on exonuclease III-assisted target recycling and hybridization chain reaction amplification strategies. Anal Methods 8(9):2106–2111
Mersal GAM, Ibrahim MM (2013) Voltammetric studies of lead at a new carbon paste microelectrode modified with N(2-isopropylphenyl)-2-thioimidazole and its trace determination in water by square wave voltammetry. Int J Electrochem Sci 8:5944–5960
Manivannan S, Ramaraj R (2009) Core–shell Au/Ag nanoparticles embedded in silicate sol–gel network for sensor application towards hydrogen peroxide. J Chem Sci 121(5):735–743
Toshima N, Yonezawa T (1998) Bimetallic nanoparticles-novel materials for chemical and physical applications. New J Chem 22(11):1179–1201
Nolan MA, Kounaves SP (1999) Microfabricated array of iridium microdisks as a substrate for direct determination of Cu2+ or Hg2+ using square-wave anodic strip** voltammetry. Anal Chem 71(16):3567–3573
Cinti S, Santella F, Moscone D, Arduini F (2016) Hg2+ detection using a disposable and miniaturized screen-printed electrode modified with nanocomposite carbon black and gold nanoparticles. Environ Sci Pollut Res 23(9):8192–8199
Yasri NG, Sundramoorthy AK, Chang WJ, Gunasekaran S (2014) Highly selective mercury detection at partially oxidized graphene/ poly (3,4-Ethylenedioxythiophene): poly (styrenesulfonate) nanocomposite film-modified electrode. Front Mater 1:1–10
Cai F, Zhu Q, Zhao K, Deng A, Li J (2015) Multiple signal amplified electrochemiluminescenct immunoassay for Hg2+ using graphene-coupled quantum dots and gold nanoparticles-labeled horse reddish peroxidase. Environ Sci Technol 49(8):5013–5020
Guha KS, Mascarenhas RJ, Thomas T, D’Souza OJ (2014) Differential pulse anodic strip** voltammetric determination of Hg2+ at poly(Eriochrome black T)-modified carbon paste electrode. Ionics 20(6):849–856
Kanchana P, Sudhan N, Anandhakumar S, Mathiyarasu J, Manisankar P, Sekar C (2015) Electrochemical detection of mercury using biosynthesized hydroxyapatite nanoparticles modified glassy carbon electrodes without preconcentration. RSC Adv 5(84):68587–68594
Gen X, Zhao H, Cken S, Quan X (2015) Electrochemical DNA sensor for specific detection of picomolar Hg(II) based on exonuclease III-assisted recycling signal amplification. Analyst. 140(6):2029–2036
Laffont L, Hezard T, Gros P, Heimbürger L-E, Sonke JE, Behra P, Evrard D (2015) Mercury(II) trace detection by a gold nanoparticle-modified glassy carbon electrode using square-wave anodic strip** voltammetry including a chloride desorption step. Talanta 141:26–32
Wu Z, Jiang L, Xu C, Ye Y, Wang X (2012) Synthesis of mesoporous NiO nanosheet and its application on mercury (II) sensor. J Solid State Electrochem 16(10):3171–3177
Zhou L, **ong W, Liu S (2015) Preparation of a gold electrode modified with au–TiO2 nanoparticles as an electrochemical sensor for the detection of mercury (II) ions. J Mater Sci 50(2):769–776
Khan Z, Obaid AY (2016) Seedless, copper-induced synthesis of stable Ag/Cu bimetallic nanoparticles and their optical properties. RSC Adv 6(35):29116–29126
Wang CY, Wang ZX, Guan J, Hu XY (2006) Voltammetric determination of meloxicam in pharmaceutical formulation and human serum at glassy carbon electrode modified by cysteic acid formed by electrochemical oxidation of l-cysteine. Sensors 6(9):1139–1152
Funding
The Higher Education Commission financially supported the study. The International Scientific Partnership Program (ISPP) at King Saud University funded of this research work through ISPP #0022.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
A facile method was developed for synthesis of bimetallic Au@Ag core-shell nanoparticles.
These nanoparticles were used as highly sensitive and selective sensor for Hg2+.
Fabricated voltammetric sensor was successfully applied for Hg2+ detection in natural water samples.
Electronic supplementary material
ESM 1
(DOCX 650 kb)
Rights and permissions
About this article
Cite this article
Siddiqui, S., Nafady, A., El-Sagher, H.M. et al. Sub-ppt level voltammetric sensor for Hg2+ detection based on nafion stabilized l-cysteine-capped Au@Ag core-shell nanoparticles. J Solid State Electrochem 23, 2073–2083 (2019). https://doi.org/10.1007/s10008-019-04298-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04298-2