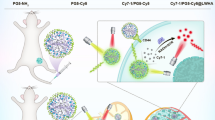
Abstract
Hypoxia, a significant feature of the tumor microenvironment, is closely associated with tumor growth, metastasis, and drug resistance. In the field of tumor microenvironment analysis, accurately imaging and quantifying hypoxia - a critical factor associated with tumor progression, metastasis, and resistance to therapy - remains a significant challenge. Herein, a hypoxia-activated red-emission fluorescent probe, ODP, for in vivo imaging of hypoxia in the tumor microenvironment is presented. Among various imaging methods, optical imaging is particularly convenient due to its rapid response and high sensitivity. The ODP probe specifically targets nitroreductase (AzoR), an enzyme highly expressed in hypoxic cells, playing a vital role by catalyzing the cleavage of azo bonds. The optical properties of ODP exhibited excellent performance in terms of fluorescence enhancement, fluorescence lifetime (0.81 ns), and detection limit (0.86 µM) in response to SDT. Cell imaging experiments showed that ODP could effectively detect and image intracellular hypoxia and the imaging capability of ODP was studied under various conditions including cell migration, antioxidant treatment, and different incubation times. Through comprehensive in vitro and in vivo experiments, including cellular imaging and mouse tumor models, this work demonstrates the efficacy of ODP in accurately detecting and imaging hypoxia. Moreover, ODP’s potential in inducing apoptosis in cancer cells offers a promising avenue for integrating diagnostic and therapeutic strategies in cancer treatment. This innovative approach not only contributes to the understanding and assessment of tumor hypoxia but also opens new possibilities for targeted cancer therapy.
Graphical Abstract







Similar content being viewed by others
Data availability
Not applicable.
References
Lee P, Chandel NS, Simon MC (2020) Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol
Pouyssegur J, Dayan F, Mazure N (2006) Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441(7092):437
Semenza L, Gregg (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10):721–732
Semenza GL (2000) HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J appl Physiol 88(4):1474–1480
Hillner B, Siegel BA, Shields AF, Liu D, Gar Ee IF, Hanna NL, Stine SH, Coleman RE (2009) The impact of positron emission tomography (PET) on expected management during cancer treatment. Findings of the National Oncologic PET Registry, Cancer
Martin DR, Danrad R, Herrmann K, Semelka RC, Hussain SM (2005) Magnetic resonance imaging of the gastrointestinal tract. Top Magn Reson Imaging Tmri 16(1):77–98
Shao Q, **ng B (2010) Photoactive molecules for applications in molecular imaging and cell biology. ChemInform 41(8):2835–2846
Zhao M Benhao, Yifan, Haisheng, **nyan, Zhang, Hongxin, Chaoran, Feng, A Tumor-Microenvironment-Responsive Lanthanide-Cyanine FRET Sensor for NIR-II; Luminescence-Lifetime In Situ Imaging of Hepatocellular Carcinoma
Zhang F, Chen Y, Pei P, Lei Z, Zhang X, Yin D (2021) A Promising NIR-II Fluorescent Sensor for Peptide‐Mediated Long‐term Monitoring of Kidney Dysfunction, Angewandte Chemie
Rauth AM, Goldberg Z, Misra V (1997) DT-Diaphorase: Possible Roles in Cancer Chemotherapy and Carcinogenesis, Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics
Sandhya S Biodegradation of Azo Dyes Under Anaerobic Condition: Role of Azoreductase, Biodegradation of Azo Dyes2010
Li X, Liang X, Yin J, Lin W Organic fluorescent probes for monitoring autophagy in living cells, Chemical Society Reviews
Jiao Y, Zhang L, Gao X, Si W, Duan C (2020) A cofactor-substrate‐based Supramolecular fluorescent probe for the Ultrafast Detection of Nitroreductase under hypoxic conditions. Angew Chem 132(15)
Thiel Z, Rivera-Fuentes P (2019) Single‐Molecule Imaging of Active Mitochondrial Nitroreductases Using a Photo‐Crosslinking Fluorescent Sensor, Angewandte Chemie International Edition
Zhang S, Chen H, Wang L, Qin X, Jiang BP, Ji SC, Shen XC, Liang H (2021) A General Approach to Design Dual Ratiometric fluorescent and photoacoustic probe for quantitatively visualizing tumor hypoxia levels in vivo. Angew Chem Int Ed Engl
Son S, Won M, Green O, Hananya N, Sharma A, Jeon Y, Kwak JH, Sessler JL, Shabat D, Kim JS (2019) Chemiluminescent probe for the InVitro and InVivo Imaging of Cancers Over-expressing NQO1. Angew Chem Int Ed
Kwon N, Cho MK, Park SJ, Kim D, Nam SJ, Cui L, Kim HM, Yoon J (2017) An efficient two-photon fluorescent probe for human NAD(P)H:quinone oxidoreductase (hNQO1) detection and imaging in tumor cells. Chem Commun 53(3):525–528
Yang YP, Qi FJ, Qian YP, Bao XZ, Zhang HC, Ma B, Dai F, Zhang SX, Zhou B (2021) Develo** Push-Pull Hydroxylphenylpolyenylpyridinium Chromophores as Ratiometric two-photon fluorescent probes for Cellular and Intravital Imaging of Mitochondrial NQO1. Anal Chem 93(4):2385–2393
Rouillere A, Johnson, Amanda E, st B, Quinn A (2017) Prasai, Bijeta, efficacious fluorescence turn-on probe for high-contrast imaging of human cells overexpressing quinone reductase activity. Chem Commun
Zhang Y, Zhao W, Chen Y, Yuan H, Fang H, Yao S, Zhang C, Xu H, Li N, Liu Z, Guo Z, Zhao Q, Liang Y, He W (2021) Rational construction of a reversible arylazo-based NIR probe for cycling hypoxia imaging in vivo. Nat Commun 12(1):2772
Wang C, Zhang S, Huang J, Cui L, Hu J, Tan S (2019) Novel designed azo substituted semi-cyanine fluorescent probe for cytochrome P450 reductase detection and hypoxia imaging in cancer cells. RSC Adv 9(37):21572–21577
**nwei T, Zhao, Li Y, Pan S, Wang H (2018) Ma, Near-Infrared Fluorescent Probes for Hypoxia Detection via Joint Regulated Enzymes: Design, Synthesis, and Application in Living Cells and Mice, Analytical Chemistry
**ang MH, Huang H, Liu XJ, Tong ZX, Zhang CX, Wang F, Yu RQ, Jiang JH (2019) Mitochondrion-Targeting Fluorescence Probe via Reduction Induced Charge Transfer for Fast Methionine Sulfoxide Reductases Imaging, Analytical Chemistry
Xu C, Zou H, Zhao Z, Zheng Z, Kwok RTK, Lam JWY, Sung HHY, Williams ID, Chen S, Zheng L (2021) B.Z. Tang, turning on Light Emission of a dark pro-aggregation‐Induced Emission Luminogen in Aqueous Media through Reductase‐Modulated Derotation. Adv NanoBiomed Res 1(4)
Yuan J, Peng R, Su D, Zhang X, Yuan L (2021) Cell membranes targeted unimolecular prodrug for programmatic photodynamic-chemo therapy. Theranostics 11(7):3502–3511
Zielonka J, Podsiad?Y R, Czerwińska M, Sikora A, Soko?Owska J, Marcinek A (2004) Color changes accompanying one-electron reduction and oxidation of the azo dyes. J Photochem Photobiology Chem 163(3):373–379
Guo Z, Park S, Yoon J, Shin I (2014) Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem Soc Rev 43(1):16–29
Beija M, Af Onso C, Martinho J (2009) Synthesis and applications of rhodamine derivatives as fluorescent probes. Chem Soc Rev
Lin Q, Bao C, Cheng S, Yang Y, Zhu L (2012) Target-activated coumarin phototriggers specifically switch on fluorescence and photocleavage upon Bonding to Thiol-Bearing protein. J Am Chem Soc 134(11):5052–5055
Wang S, Ren WX, Hou JT, Won M, Kim JS (2021) Fluorescence imaging of pathophysiological microenvironments. Chem Soc Rev 50(4)
Owens EA, Henary M, Fakhri GE, Choi HS (2016) Tissue-Specific Near-Infrared Fluorescence Imaging, Accounts of Chemical Research 1731
Tian Y, Li Y, Jiang W-L, Zhou D-Y, Fei J, Li C-Y (2019) In-situ imaging of azoreductase activity in the acute and chronic ulcerative colitis mice by a near-infrared fluorescent probe. Anal Chem 91(16):10901–10907
Piao W, Tsuda S, Tanaka Y, Maeda S, Liu F, Takahashi S, Kushida Y, Komatsu T, Ueno T, Terai T, Nakazawa T, Uchiyama M, Morokuma K, Nagano T, Hanaoka K (2013) Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew Chem Int Ed Engl 52(49):13028–13032
Funding
This research was supported by grants of Postdoctoral Science Foundation of China (No. 2019M651781 and 2019TQ0142) and the Innovation Fund for Technology of Nan**g University (2021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All animal care and experimental protocols complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China and were approved by the Model Animal Research Center (MARC) of Nan**g University.
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
**, C., Wu, P., Tu, M. et al. Development of a hypoxia-activated red-emission fluorescent probe for in vivo tumor microenvironment imaging and anti-tumor therapy. Microchim Acta 191, 217 (2024). https://doi.org/10.1007/s00604-024-06291-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06291-7