Abstract
A triple recognition voltammetric method for the determination of brain natriuretic peptide (BNP) is described. Gold nanoparticles (AuNPs) and magnetic nanoparticles (MagNPs), sized 26 and 310 nm, respectively, were synthesized and characterized by transmission electron microscopy (TEM), FT-IR, dynamic light scattering (DLS), and Z-potential measurements. Antibody-modified MagNPs and methylene blue–labeled aptamer (Apt-MB)–modified AuNPs were used as an identifier, a signal reporter, and an amplifier, respectively. In the presence of BNP, the magnetic gold nanocomposite is formed through cascade conjugation via specific interaction. It then hybridized with complementary DNA (cDNA) on the interface, thereby amplifying the current signal of Apt-MB and increasing the selectivity of the immunoassay. Results obtained demonstrate the development of a highly selective method with a detection limit of 0.56 pg mL−1 and a linear response over the concentration range 1–10,000 pg mL−1. The standard deviation of the method is < 6% while the recovery ranged from 92.2 to 104.2%.

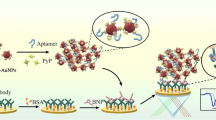
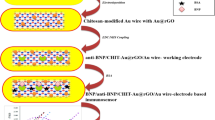
Schematic representation of triple recognition electrochemical immunosensor based on two functionalized nanoparticles (antibody-modified magnetic nanoparticle (MNP-Ab) and aptamer-modified gold nanoparticle (AuNPs-Apt)) for determination of brain natriuretic peptide (BNP).





Similar content being viewed by others
References
Sarangadharan I, Wang SL, Tai TY, Pulikkathodi AK, Hsu CP, Chiang HHK, Wang YL (2018) Risk stratification of heart failure from one drop of blood using hand-held biosensor for BNP detection. Biosens Bioelectron 107:259–265. https://doi.org/10.1016/j.bios.2018.02.036
Lin DCC, Diamandis EP, Januzzi JL, Maisel A, Jaffe AS, Clerico A (2014) Natriuretic peptides in heart failure. Clin Chem 60:1040–1046. https://doi.org/10.1373/clinchem.2014.223057
Li X, Liu L, Dong X, Zhao G, Li Y, Miao J, Fang J, Cui M, Wei Q, Cao W (2019) Dual mode competitive electrochemical immunoassay for B-type natriuretic peptide based on GS/SnO2/polyaniline-Au and ZnCo2O4/N-CNTs. Biosens Bioelectron 126:448–454. https://doi.org/10.1016/j.bios.2018.11.009
Serafín V, Torrente-Rodríguez RM, González-Cortés A, García de Frutos P, Sabaté M, Campuzano S, Yáñez-Sedeño P, **arróna JM (2018) An electrochemical immunosensor for brain natriuretic peptide prepared with screen-printed carbon electrodes nanostructured with gold nanoparticles grafted through aryl diazonium salt chemistry. Talanta 179:131–138. https://doi.org/10.1016/j.talanta.2017.10.063
Szunerits S, Mishyn V, Grabowska I, Boukherroub R (2019) Electrochemical cardiovascular platforms: current state of the art and beyond. Biosens Bioelectron 131:287–298. https://doi.org/10.1016/j.bios.2019.02.010
Nishikimi T, Okamoto H, Nakamura M, Ogawa N, Horii K, Nagata K, Nakagawa Y, Kinoshita H, Yamada C (2013) Direct immunochemiluminescent assay for proBNP and total BNP in human plasma proBNP and total BNP levels in normal and heart failure. PLoS One 8:e53233. https://doi.org/10.1371/journal.pone.0053233
Jang HR, Wark AW, Baek SH, Chung BH, Lee HJ (2014) Ultrasensitive and ultrawide range detection of a cardiac biomarker on a surface plasmon resonance platform. Anal Chem 86:814–819. https://doi.org/10.1021/ac4033565
Kim S, Wark AW, Lee HJ (2016) Femtomolar detection of tau proteins in undiluted plasma using surface plasmon resonance. Anal Chem 88:7793–7799. https://doi.org/10.1021/acs.analchem.6b01825
Zhao Y, Li L, Hu L, Zhang Y, Wu D, Ma H, Wei Q (2019) An electrochemiluminescence immunosensor for the N-terminal brain natriuretic peptide based on the high quenching ability of polydopamine. Microchim Acta 186:606. https://doi.org/10.1007/s00604-019-3709-x
Lim MJ, Foster GJ, Gite S, Ostendorff HP, Narod S, Rothschild KJ (2010) An ELISA-based high throughput protein truncation test for inherited breast cancer. Breast Cancer Res 12:78–79. https://doi.org/10.1186/bcr2722
Auld D, Lea W, Davis MI, Simeonov A (2013) Literature search and review: detected through a quick glance. Assay Drug Dev Techn 11:1–8. https://doi.org/10.1089/adt.2013.1101.lr
Toh SY, Citartan M, Gopinath SC, Tang TH (2015) Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens Bioelectron 64:392–403. https://doi.org/10.1016/j.bios.2014.09.026
Wang P, Hatcher KL, Bartz JC, Chen SG, Skinner P, Sreevatsan S (2011) Selection and characterization of DNA aptamers against PrPSc. Exp Biol Med 236:466–476. https://doi.org/10.1258/ebm.2011.010323
Shamah SM, Healy JM, Cload ST (2008) Complex target SELEX. Acc Chem Res 41:130–138. https://doi.org/10.1021/ar700142z
Kim SH, Nam O, ** E, Gu MB (2019) A new coccolith modified electrode-based biosensor using a cognate pair of aptamers with sandwich-type binding. Biosens Bioelectron 123:160–166. https://doi.org/10.1016/j.bios.2018.08.021
Lin Y, Wang X, Sun Y, Dai Y, Sun W, Zhu X, Luo C (2019) A chemiluminescent biosensor for ultrasensitive detection of adenosine based on target-responsive DNA hydrogel with Au@HKUST-1 encapsulation. Sensors Actuators B Chem 289:56–64. https://doi.org/10.1016/j.snb.2019.03.075
Zhang W, He Z, Yi L, Mao S, Li H, Lin JM (2018) A dual-functional microfluidic chip for on-line detection of interleukin-8 based on rolling circle amplification. Biosens Bioelectron 102:652–660. https://doi.org/10.1016/j.bios.2017.12.017
Yoon J, Cho SH, Seong H (2017) Multifunctional ultrasmall superparamagnetic iron oxide nanoparticles as a theranostic agent. Colloid Surface A 520:892–902. https://doi.org/10.1016/j.colsurfa.2017.02.080
Melnychuk N, Klymchenko AS (2018) DNA-functionalized dye-loaded polymeric nanoparticles: ultrabright FRET platform for amplified detection of nucleic acids. J Am Chem Soc 140:10856–10865. https://doi.org/10.1021/jacs.8b05840
Zheng L, Qi P, Zhang D (2018) A simple, rapid and cost-effective colorimetric assay based on the 4-mercaptophenylboronic acid functionalized silver nanoparticles for bacteria monitoring. Sensors Actuators B Chem 260:983–989. https://doi.org/10.1016/j.snb.2018.01.115
Lv S, Sheng J, Zhao S, Liu M, Chen L (2018) The detection of brucellosis antibody in whole serum based on the low-fouling electrochemical immunosensor fabricated with magnetic Fe3O4@Au@PEG@HA nanoparticles. Biosens Bioelectron 117:138–144. https://doi.org/10.1016/j.bios.2018.06.010
Tavallaie R, McCarroll J, Le Grand M, Ariotti N, Schuhmann W, Bakker E, Gooding JJ (2018) Nucleic acid hybridization on an electrically reconfigurable network of gold-coated magnetic nanoparticles enables microRNA detection in blood. Nat Nanotechnol 13:1066–1071. https://doi.org/10.1038/s41565-018-0232-x
Tetin SY, Ruan Q, Saldana SC, Pope MR, Chen Y, Wu H, Richardson PL (2006) Interactions of two monoclonal antibodies with BNP: high resolution epitope map** using fluorescence correlation spectroscopy. Biochemistry 45:14155–14165. https://doi.org/10.1021/bi0607047
Wang Y, Wu J, Chen Y, Xue F, Teng J, Cao J, Chen W (2015) Magnetic microparticle-based SELEX process for the identification of highly specific aptamers of heart marker--brain natriuretic peptide. Microchim Acta 182:331–339. https://doi.org/10.1007/s00604-014-1338-y
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of Au colloid monolayers. Anal Chem 67:735–743. https://doi.org/10.1021/ac00100a008
Park JW, Shumaker-Parry JS (2014) Structural study of citrate layers on gold nanoparticles: role of intermolecular interactions in stabilizing nanoparticles. J Am Chem Soc 136:1907–1921. https://doi.org/10.1021/ja4097384
Huerta-Nuñez LFE, Gutierrez-Iglesias G, Martinez-Cuazitl A, Mata-Miranda MM, González-Díaz CA (2019) A biosensor capable of identifying low quantities of breast cancer cells by electrical impedance spectroscopy. Sci Rep 9:6419. https://doi.org/10.1038/s41598-019-42776-9
Fan T, Du Y, Yao Y, Wu J, Meng S, Luo J, Gao F (2018) Rolling circle amplification triggered poly adenine-gold nanoparticles production for label-free electrochemical detection of thrombin. Sensors Actuators B Chem 266:9–18. https://doi.org/10.1016/j.snb.2018.03.112
Grabowska I, Sharma N, Vasilescu A, Iancu M, Badea G, Boukherroub R, Ogale S, Szunerits S (2018) Electrochemical aptamer-based biosensors for the detection of cardiac biomarkers. ACS Omega 3:12010–12018. https://doi.org/10.1021/acsomega.8b01558
Lee I, Luo X, Huang J, Cui XT, Yun M (2012) Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors 2:205–220. https://doi.org/10.3390/bios2020205
Xu R, Lu P, Wu B, Wang X, Pang X, Du B, Fan D, Wei Q (2018) Using SiO2/PDA-Ag NPs to dual-inhibited photoelectrochemical activity of CeO2-CdS composites fabricated a novel immunosensor for BNP ultrasensitive detection. Sensors Actuators B Chem 274:349–355. https://doi.org/10.1016/j.snb.2018.07.122
Lei YM, **ao MM, Li YT, Xu L, Zhang H, Zhang ZY, Zhang GJ (2017) Detection of heart failure-related biomarker in whole blood with graphene field effect transistor biosensor. Biosens Bioelectron 91:1–7. https://doi.org/10.1016/j.bios.2016.12.018
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 61275085).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 872 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Zhu, ZZ., Huang, X. et al. Magnetic gold nanocomposite and aptamer assisted triple recognition electrochemical immunoassay for determination of brain natriuretic peptide. Microchim Acta 187, 231 (2020). https://doi.org/10.1007/s00604-020-4221-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4221-z