Abstract
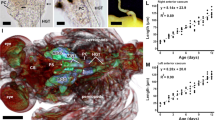
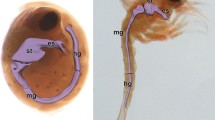
Arthropods are the most diversified animals on Earth. The morphology of the digestive system has been widely studied in insects; however, crustaceans have received comparatively little attention. This study describes the hindgut tract of the common spider crab Maja brachydactyla Balss, 1922, in larvae and adults using dissection, light and electron microscopical analyses. The hindgut tract maintains a similar general shape in larvae and adults. Major differences among stages are found in the morphology of epithelial cells and microspines, the thickness of the cuticle and connective-like tissue, and the presence of rosette glands (only in adults). Here, we provide the description of the sub-cellular structure of the folds, epithelium (conformed by tendon cells), musculature, and microspines of the hindgut of larvae and adults of M. brachydactyla. The morphological features of the hindgut of M. brachydactyla are compared with those of other arthropods (Insecta, Myriapoda and Arachnida). Our results suggest that the morphology of the hindgut is associated mainly with transport of faeces. In adults, the hindgut may also exert an osmoregulatory function, as described in other arthropods. At difference from holometabolous insets, the hindgut of M. brachydactyla (Decapoda) does not undergo a true metamorphic change during development, but major changes observed between larval and adult stages might respond to the different body size between life stages.








Similar content being viewed by others
References
Abelló P, García Raso JE, Guerao G, Salmerón F (2014) Maja brachydactyla (Brachyura: Majidae) in the western Mediterranean. Mar Biodivers Rec 7:
Abrunhosa FA, Kittaka J (1997) Morphological changes in the midgut, midgut gland and hindgut during the larval and postlarval development of the red king crab Paralithodes camtschaticus. Fish Sci 63:746–754
Alexander CG (1989) Tegumental glands in the paragnaths of Palaemon serratus (Crustacea: Natantia). J Mar Biol Assoc U K 69:53–63
Allardyce BJ, Linton SM (2010) Functional morphology of the gastric mills of carnivorous, omnivorous, and herbivorous land crabs. J Morphol 271:61–72
Andrés M, Estévez A, Anger K, Rotllant G (2008) Developmental patterns of larval growth in the edible spider crab, Maja brachydactyla (Decapoda: Majidae). J Exp Mar Biol Ecol 357:35–40
Andrés M, Estévez A, Rotllant G (2007) Growth, survival and biochemical composition of spider crab Maja brachydactyla (Balss, 1922) (Decapoda: Majidae) larvae reared under different stocking densities, prey:larva ratios and diets. Aquaculture 273:494–502
Andrés M, Estévez A, Simeó CG, Rotllant G (2010) Annual variation in the biochemical composition of newly hatched larvae of Maja brachydactyla in captivity. Aquaculture 310:99–105
Areekul S (1957) The comparative internal larval anatomy of several genera of Scarabaeidae (Coleoptera)1. Ann Entomol Soc Am 50:562–577
Barker PL, Gibson R (1977) Observations on the feeding mechanism, structure of the gut, and digestive physiology of the european lobster Homarus gammarus (L.) (Decapoda: Nephropidae). J Exp Mar Biol Ecol 26:297–324
Barker PL, Gibson R (1978) Observations on the structure of the mouthparts, histology of the alimentary tract, and digestive physiology of the mud crab Scylla serrata (Forskål) (Decapoda: Portunidae). J Exp Mar Biol Ecol 32:177–196
Beadle DJ (1973) Muscle attachment in the tick, Boophilus decoloratus Koch (Acarina : Ixodidae). Int J Insect Morphol Embryol 2:247–255
Bitsch C, Bitsch J (2002) The endoskeletal structures in arthropods: cytology, morphology and evolution. Arthropod Struct Dev 30:159–177
Bogataj U, Praznik M, Mrak P, Štrus J, Tušek-Žnidarič M, Žnidaršič N (2018) Comparative ultrastructure of cells and cuticle in the anterior chamber and papillate region of Porcellio scaber (Crustacea, Isopoda) hindgut. ZooKeys 427
Byers JR, Bond EF (1971) Surface specializations of the hindgut cuticle of lepidopterous larvae. Can J Zool 49:867–876
Castejón D, Alba-Tercedor J, Rotllant G, Ribes E, Durfort M, Guerao G (2018) Micro-computed tomography and histology to explore internal morphology in decapod larvae. Sci Rep 8:14399
Castejón D, Rotllant G, Alba-Tercedor J, Font-i-Furnols M, Ribes E, Durfort M, Guerao G (2019a) Morphology and ultrastructure of the midgut gland (“hepatopancreas”) during ontogeny in the common spider crab Maja brachydactyla Balss, 1922 (Brachyura, Majidae). Arthropod Struct Dev 49:137–151
Castejón D, Rotllant G, Giménez L, Torres G, Guerao G (2018b) Influence of temperature and light regime on the larval development of the common spider crab Maja brachydactyla Balss, 1922 (Brachyura: Majidae). Aquac Res 49:3548–3558
Castejón D, Rotllant G, Guerao G (2019b) Factors influencing successful settlement and metamorphosis of the common spider crab Maja brachydactyla Balss, 1922 (Brachyura: Majidae): Impacts of larval density, adult exudates and different substrates. Aquaculture 501:374–381
Castejón D, Rotllant G, Ribes E, Durfort M, Guerao G (2015) Foregut morphology and ontogeny of the spider crab Maja brachydactyla (Brachyura, Majoidea, Majidae). J Morphol 276:1109–1122
Castejón D, Rotllant G, Ribes E, Durfort M, Guerao G (2018c) Morphology and ultrastructure of the esophagus during the ontogeny of the spider crab Maja brachydactyla (Decapoda, Brachyura, Majidae). J Morphol 279:710–723
Castejón D, Rotllant G, Ribes E, Durfort M, Guerao G (2019c) Structure of the stomach cuticle in adult and larvae of the spider crab Maja brachydactyla (Brachyura, Decapoda). J Morphol 280:370–380
Cazemier AE, Hackstein JHP, Op den Camp HJM, Rosenberg J, van der Drift C (1997) Bacteria in the intestinal tract of different species of arthropods. Microb Ecol 33:189–197
Ceccaldi HJ (1989) Anatomy and physiology of digestive tract of Crustaceans Decapods reared in aquaculture. Advances in Tropical Aquaculture, Workshop at Tahiti, French Polynesia, vol 9. Actes de colloques Ifremer, Tahiti, French Polynesia, pp 243–259
Chisaka H, Ueno M, Futaesaku Y (1999) Spines in the hindgut of the crayfish Procambarus clarkii (Decapoda): their distribution and correlation with hindgut muscles. J Crust Biol 19:337–343
Coleman CO (1991) Comparative fore-gut morphology of Antarctic Amphipoda (Crustacea) adapted to different food sources. Hydrobiologia 223:1–9
Coleman CO (1992) Foregut morphology of amphipoda (crustacea). An example of its relevance for systematics. Ophelia 36:135–150
Coleman CO (1994) Comparative anatomy of the alimentary canal of hyperiid amphipods. J Crust Biol 14:346–370
Corgos A, Bernárdez C, Sampedro P, Verísimo P, Freire J (2011) Spatial structure of the spider crab, Maja brachydactyla population: evidence of metapopulation structure. J Sea Res 66:9–19
Dapples CC, Lea AO (1974) Inner surface morphology of the alimentary canal in Aedes aegypti (L.) (diptera: culicidae). Int J Insect Morphol Embryol 3:433–442. https://www.sciencedirect.com/science/article/abs/pii/002073227490035X
Davie PJF, Guinot D, Ng PKL (2015) Anatomy and functional morphology of Brachyura. In: Castro P, Davie PJF, Guinot D, Schram F, Von Vaupel Klein C (eds) Treatise on Zoology - Anatomy Taxonomy Biology. The Crustacea Volume 9 Part C. Brill, pp 11–163
De Jong L, Casanova B (1997) Comparative morphology of the foregut of three Eucopia species (Crustacea, Mysidacea, Lophogastrida). J Nat Hist 31:389–402. https://www.tandfonline.com/doi/abs/10.1080/00222939700770191
Dewel RA, Dewel WC (1979) Studies on the tardigrades. IV. Fine structure of the hindgut of Milnesium tardigradum doyère. J Morphol 161:79–109
Diaz E, Cisneros R, ZuñIga G, Uria-Galicia E (1998) Comparative anatomical and histological study of the alimentary canal of Dendroctonus parallelocollis, D. rhizophagus, and D. valens (Coleoptera: Scolytidae). Ann Entomol Soc Am 91:479–487
Dillaman RM, Roer R, Shafer T, Modla S (2013) The crustacean integument: structure and function. In: Watling L, Thiel M (eds) The Natural History of the Crustacea, vol. 1. Functional Morphology and Diversity, vol Volume 1. Oxford University Press, pp 140–165
Doughtie DG, Rao KR (1982) Rosette glands in the gills of the grass shrimp, Palaemonetes pugio. I. Comparative morphology, cyclical activity, and innervation. J Morphol 171:41–67
Dworschak PC (1998) The role of tegumental glands in burrow construction of two Mediterranean callianassid shrimp. Senckenb Marit 28:143–149
Elzinga RJ (1996) A comparative study of microspines in the alimentary canal of five families of Orthoptera (Saltatoria). Int J Insect Morphol Embryol 25:249–260. https://www.sciencedirect.com/science/article/abs/pii/0020732296000074
Elzinga RJ (1998) Microspines in the alimentary canal of arthropoda, onychophora, annelida. Int J Insect Morphol Embryol 27:341–349
Elzinga RJ, Hopkins TL (1994) Foregut microspines in four families of cockroaches (Blattaria). Int J Insect Morphol Embryol 23:253–260. https://www.sciencedirect.com/science/article/abs/pii/0020732294900221
Elzinga RJ, Hopkins TL (1995) Microspine variation in hindgut regions of four families of cockroaches (Blattaria). Int J Insect Morphol Embryol 24:203–211
Erri Babu D, Shyamasundari K, Hanumantha Rao K (1979) The structure and histochemistry of the oesophageal glands in the crab Menippe rumphii (Frabricius) (Crustacea : Brachyura). Proc Anim Sci 88:277–285
Erri Babu D, Shyamasundari K, Rao KH (1982) Studies on the digestive system of the crab Menippe rumphii (Fabricius) (Crustacea:Brachyura). J Exp Mar Biol Ecol 58:175–191
Factor JR (1981) Development and metamorphosis of the digestive system of larval lobsters, Homarus americanus (Decapoda: Nephropidae). J Morphol 169:225–242
Felder DL, Felgenhauer BE (1993) Morphology of the midgut–hindgut juncture in the ghost shrimp Lepidophthalmus louisianensis (Schmitt) (Crustacea: Decapoda: Thalassinidea). Acta Zool 74:263–276
Felgenhauer BE (1992) Chapter 3. Internal anatomy of the Decapoda: an overview. In: Harrison FW, Humes AG (eds) Microscopic Anatomy of Invertebrates. Volume 10: Decapod Crustacea, vol 10, Decapod Crustacea. Wiley-Liss, Inc., pp 45–75
Forsythe TG (1982) Feeding mechanisms of certain ground beetles (Coleoptera: Carabidae). Coleopt Bull 26–73
Freire J, Bernárdez C, Corgos A, Fernández L, González-Gurriarán E, Sampedro MP, Verísimo P (2002) Management strategies for sustainable invertebrate fisheries in coastal ecosystems of Galicia (NW Spain). Aquat Ecol 36:41–50
Friesen JA, Mann KH, Willison JHM (1986) Gross anatomy and fine structure of the gut of the marine mysid shrimp Mysis stenolepis Smith. Can J Zool 64:431–441. https://cdnsciencepub.com/doi/10.1139/z86-067
Gonçalves WG, Fernandes KM, Santana WC, Martins GF, Zanuncio JC, Serrão JE (2017) Post-embryonic changes in the hindgut of honeybee Apis mellifera workers: morphology, cuticle deposition, apoptosis, and cell proliferation. Dev Biol 431:194–204
González-Gurriarán E, Fernández L, Freire J, Muiño R, Parapar J (1993) Reproduction of the spider crab Maja squinado (Brachyura: Majidae) in the southern Galician coast (NW Spain). International Council for the Exploration of the Sea. Shellfish Committe ICES C.M. 1993/K:19
González-Gurriarán E, Freire J, Parapar J, Sampedro MP, Urcera M (1995) Growth at moult and moulting seasonality of the spider crab, Maja squinado (Herbst) (Decapoda: Majidae) in experimental conditions: implications for juvenile life history. J Exp Mar Biol Ecol 189:183–203
Green TL (1933) Some aspects of the metamorphosis of the alimentary system in the wasp, Vespa vulgaris (Hymenoptera). Proc Zool Soc London 103:629–644
Guerao G, Pastor E, Martin J, Andrés M, Estévez A, Grau A, Duran J, Rotllant G (2008) The larval development of Maja squinado and M. brachydactyla (Decapoda, Brachyura, Majidae) described from plankton collected and laboratory-reared material. J Nat Hist 42:2257–2276
Guerao G, Rotllant G (2010) Development and growth of the early juveniles of the spider crab Maja squinado (Brachyura: Majoidea) in an individual culture system. Aquaculture 307:105–110
Guerao G, Rotllant G, Anger K (2010) Characterization of larval moulting cycles in Maja brachydactyla (Brachyura, Majidae) reared in the laboratory. Aquaculture 302:106–111
Günzl H (1991) The ultrastructure of the posterior gut and caecum in Alona affinis (Crustacea, Cladocera). Zoomorphology 110:139–144
Harris J (1993a) The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microb Ecol 25:195–231
Harris JM (1993b) Widespread occurrence of extensive epimural rod bacteria in the hindguts of marine Thalassinidae and Brachyura (Crustacea: Decapoda). Mar Biol 116:615–629
Heeren T, Mitchell BD (1997) Morphology of the mouthparts, gastric mill and digestive tract of the giant crab, Pseudocarcinus gigas (Milne Edwards) (Decapoda: Oziidae). Mar Freshw Res 48:7–18
Herrera-Alvarez L, Fernández I, Benito J, Pardos F (2000) Ultrastructure of the midgut and hindgut of Derocheilocaris remanei (Crustacea, Mystacocarida). J Morphol 244:177–189
Hinton DJ, Corey S (1979) The mouthparts and digestive tract in the larval stages of Homarus americanus. Can J Zool 57:1413–1423
Hochuli DF, Roberts B, Sanson GD (1992) Anteriorly directed microspines in the foregut of Locusta migratoria (Orthoptera : Acrididae). Int J Insect Morphol Embryol 21:95–97. https://www.sciencedirect.com/science/article/abs/pii/002073229290008B
Holdich DM, Mayes KR (1975) A fine-structural re-examination of the so-called ‘midgut’ of the isopod Porcellio. Crustaceana 29:186–192
Holdich DM, Ratcliffe NA (1970) A light and electron microscope study of the hindgut of the herbivorous isopod, Dynamene bidentata (Crustacea: Peracarida). Z Zellforsch Mikrosk Anat 111:209–227
Hopkin SP, Nott JA (1980) Studies on the digestive cycle of the shore crab Carcinus maenas (L.) with special reference to the b cells in the hepatopancreas. J Mar Biol Assoc UK 60:891–907
Hoyoux C, Zbinden M, Samadi S, Gaill F, Compère P (2009) Wood-based diet and gut microflora of a galatheid crab associated with Pacific deep-sea wood falls. Mar Biol 156:2421–2439. https://springer.longhoe.net/article/10.1007/s00227-009-1266-2
Icely JD, Nott JA (1984) On the morphology and fine structure of the alimentary canal of Corophium volutator (Pallas) (Crustacea: Amphipoda). Philos Trans R Soc Lond B Biol Sci 306:49–78. https://royalsocietypublishing.org/doi/10.1098/rstb.1984.0081
Icely JD, Nott JA (1992) Chapter 6. Digestion and absorption: digestive system and associated organs. In: Harrison FW, Humes AG (eds) Microscopic Anatomy of Invertebrates. Volume 10: Decapod Crustacea, vol 10, Decapod Crustacea. Wiley-Liss, Inc., pp 45–75
Jantrarotai PN, Sawanyatiputi SA (2005) Histological study on the development of digestive system in zoeal stages of mud crab (Scylla olivacea). Kasetsart J 39:666–671
Jones JC (1960) The anatomy and rhythmical activities of the alimentary canal of Anopheles larvae. Ann Entomol Soc Am 53:459–474
Johnston DJ, Alexander CG (1999) Functional morphology of the mouthparts and alimentary tract of the slipper lobster Thenus orientalis (Decapoda : Scyllaridae). Mar Freshw Res 50:213–223. https://www.publish.csiro.au/mf/MF98089
Johnston M, Johnston D, Knott B (2008) Ontogenetic changes in the structure and function of the mouthparts and foregut of early and late stage Panulirus ornatus (Fabricius, 1798) phyllosomata (Decapoda: Palinuridae). J Crust Biol 28:46–56. https://academic.oup.com/jcb/article/28/1/46/2548279
Johnston MD, Johnston DJ, Richardson AMM (2004) Mouthpart and digestive tract structure in four talitrid amphipods from a translittoral series in Tasmania. J Mar Biolog Assoc UK 84:717–726. https://doi.org/10.1017/S0025315404009804h
Judy KJ, Gilbert LI (1969) Morphology of the alimentary canal during the metamorphosis of Hyalophora cecropia (Lepidoptera: Saturniidae)1. Ann Entomol Soc Am 62:1438–1446
Klann AE, Alberti G (2010) Histological and ultrastructural characterization of the alimentary system of solifuges (Arachnida, Solifugae). J Morphol 271:225–243
Klowden MJ (2013) Chapter 6 - metabolic systems. Physiological Systems in Insects, Third Edition. Academic Press, San Diego, pp 305–364
Komuro T, Yamamoto T (1968) Fine structure of the epithelium of the gut in the crayfish (Procambarus clarkii) with special reference to the cytoplasmic microtutubles. Arch Histol Jpn 30:17–32
Lovett DL, Felder DL (1989) Ontogeny of gut morphology in the white shrimp Penaeus setiferus (Decapoda, Penaeidae). J Morphol 201:253–272
Mall SB (1980) Histomorphology of the alimentary canal and associated glands of the mature larva of Marasmia trapezalis Guen. (Pyralidae: Lepidoptera). J Nat Hist 14:97–110
Martin JW, Olesen J, Høeg JT (2014) Atlas of Crustacean Larvae. Johns Hopkins University Press, Baltimore
Mathieson BRF, Lehane MJ (2002) Ultrastructure of the alimentary canal of the sheep scab mite, Psoroptes ovis (Acari: Psoroptidae). Vet Parasitol 104:151–166
Mathur LML (1973) Histology of the alimentary canal of the mature larva of Prodenia litura Fabr. (Lepidoptera). J Nat Hist 7:653–664
Maxwell DE (1955) The comparative internal larval anatomy of sawflies (Hymenoptera: Symphyta). Memoirs of the Entomological Society of Canada 87:5–132
McLaughlin PA (1983) 1. Internal anatomy. In: Mantel LH (ed) The Biology of Crustacea Vol. 5. Internal Anatomy and Physiological Regulation, vol Vol. 5. Academic Press, pp 1–52
Metillo EB, Ritz DA (1994) Comparative foregut functional morphology of three co-occurring mysids (Crustacea: Mysidacea) from south-eastern Tasmania. JMBA-J Mar Biol Assoc UK 74:323–336
Mikami S, Greenwood JG, Takashima F (1994) Functional morphology and cytology of the phyllosomal digestive system of Ibacus ciliatus and Panulirus japonicus (Decapoda, Scyllaridae and Palinuridae). Crustaceana 67:212–225
Miyoshi AR, Gabriel VA, Fantazzini ER, Fontanetti CS (2005) Microspines in the pylorus of Pseudonannolene tricolor and Rhinocricus padbergi (Arthropoda, Diplopoda). Iheringia Série Zoologia 95:183–187
Moon YW, Kim HH (1999) Morphological study of the digestive tract of the mud crab (Hemigrapsus penicillatus De Haan) and the symbiotic crab (Pinnotheres cyclinus Shen). Korean J Biol Sci 3:407–412
Mykles DL (1979) Ultrastructure of alimentary epithelia of lobsters, Homarus americanus and H. gammarus, and crab. Cancer Magister Zoomorphologie 92:201–215
Nakazawa E, Katoh K, Ishikawa H (1992) The association of microtubules with the plasmalemma in epidermal tendon cells of the river crab. Biol Cell 75:111–119
Nardi JB, Bee CM, Miller LA, Nguyen NH, Suh S-O, Blackwell M (2006) Communities of microbes that inhabit the changing hindgut landscape of a subsocial beetle. Arthropod Struct Dev 35:57–68
Nardi JB, Bee CM, Miller LA, Taylor SJ (2009) Distinctive features of the alimentary canal of a fungus-feeding hemipteran, Mezira granulata (Heteroptera: Aradidae). Arthropod Struct Dev 38:206–215
Nardi JB, Bee CM, Taylor SJ (2016) Compartmentalization of microbial communities that inhabit the hindguts of millipedes. Arthropod Struct Dev 45:462–474
ØDegaard F, (2000) How many species of arthropods? Erwin’s estimate revised. Biol J Lin Soc 71:583–597
Pazos G, Fernández J, Linares F, Sánchez J, Otero JJ, Iglesias J, Domingues P (2018) The complete life cycle in captivity of the spider crab, Maja brachydactyla, Herbst 1788. Aquac Res 0:
Phillips JE, Hanrahan J, Chamberlin M, Thomson B (1987) Mechanisms and control of reabsorption in insect hindgut. In: Evans PD, Wigglesworth VB (eds) Advances in Insect Physiology, vol 19. Academic Press, pp 329–422
Pillai RS (1960) Studies on the shrimp Caridina laevis (Heller) 1. The Digestive System. J Mar Biol Ass India 2:57–74. http://mbai.org.in/uploads1/manuscripts/Article%202%20(172-176)330539914.pdf
Pinn EH, Nickell LA, Rogerson A, Atkinson RJA (1999) Comparison of gut morphology and gut microflora of seven species of mud shrimp (Crustacea: Decapoda: Thalassinidea). Mar Biol 133:103–114. https://springer.longhoe.net/article/10.1007/s002270050448
Potts SF (1927) The alimentary canal of the Mexican bean beetle. Ohio J Sci 27:127–137
Pugh JE (1962) A contribution toward a knowledge of the hind-gut of fiddler crabs (Decapoda, Grapsidae). Trans Am Microsc Soc 81:309–320
Reddy AR (1937) The physiology of digestion and absorption in the crab Paratelphusa (Oziotelphusa) hydrodromus (Herbst). Proc Indiana Acad Sci - Section B 6:170–193
Reedy MC, Beall C (1993) Ultrastructure of develo** flight muscle in Drosophila. II. Formation of the myotendon junction. Dev Biol 160:466–479
Richins CA (1938) The metamorphosis of the digestive tract of Aedes dorsalis Meigen. Ann Entomol Soc Am 31:69–73
Rowland IJ, Goodman WG (2016) Magnetic resonance imaging of alimentary tract development in Manduca sexta. PLoS ONE 11:e0157124
Santos HP, Rost-Roszkowska M, Vilimova J, Serrão JE (2017) Ultrastructure of the midgut in Heteroptera (Hemiptera) with different feeding habits. Protoplasma 254:1743–1753
Schlegel C (1911) Anatomie sommaire de la première zoé de Maja squinado Latr. (Note préliminaire à des recherches sur l'Organogénese des Décapodes brachyoures). Archives de Zoologie Experimentale et Générale 5º Série T. VIII.:29–40
Schmitz EH, Scherrey PM (1983) Digestive anatomy Hyalella azteca (Crustacea, Amphipoda). J Morphol 175:91–100
Schultz TW, Kennedy JR (1976) The fine structure of the digestive system of Daphnia pulex (Crustacea: Cladocera). Tissue Cell 8:479–490
Shelomi M, Sitepu IR, Boundy-Mills KL, Kimsey LS (2015) Review of the gross anatomy and microbiology of the phasmatodea digestive tract. J Orthop Res 24(29–40):12
Schlüter U (1980) Ultrastruktur der Pyloruszähnchen zweier Tausendfüßler (Tachypodoiulus niger, Polydesmus angustus)*. Acta Zool 61:171–178. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1463-6395.1980.tb01305.x
Simeó CG, Andrés M, Estévez A, Rotllant G (2015) The effect of male absence on the larval production of the spider crab Maja brachydactyla Balss, 1922. Aquac Res 46:937–944
Smit W, Akster H (1974) Bridges between microtubules and desmosomes in the terminal filament-cuticle connection and in the muscle-cuticle connection (tendon cell) of the Colorado beetle (Leptinotarsa decemlineata Say). Netherlands J Zool 25:122–124
Smith D, Järlfors U, Russell F (1969) The fine structure of muscle attachments in a spider (Latrodectus mactans, Fabr.). Tissue Cell 1:673–687
Storch V, Strus J, Brandt A (2002) Microscopic anatomy and ultrastructure of the digestive system of Natatolana obtusata (Vanhöffen, 1914) (Crustacea, Isopoda). Acta Zool 83:1–14. https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1463-6395.2002.00093
Stork NE, McBroom J, Gely C, Hamilton AJ (2015) New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. Proc Natl Acad Sci 112:7519–7523
Talarico G, Lipke E, Alberti G (2011) Gross morphology, histology, and ultrastructure of the alimentary system of Ricinulei (Arachnida) with emphasis on functional and phylogenetic implications. J Morphol 272:89–117
Talbot P, Al-Hajj H, Demers D, Howard D (1991) Distribution of microfilaments and microtubules in Homarus pleopod tegumental glands (Crustacea, Decapoda). Zoomorphology 110:329–338
Terra WR (1990) Evolution of digestive systems of insects. Annu Rev Entomol 35:181–200
Terra WR, Ferreira C (2020) Evolutionary trends of digestion and absorption in the major insect orders. Arthropod Struct Dev 56:100931
To TH, Brenner TL, Cavey MJ, Wilkens JL (2004) Histological organization of the intestine in the crayfish Procambarus clarkii. Acta Zool 85:119–130
Trinadha Babu B, Shyamasundari K, Hanumantha Rao K (1989) Observations on the morphology and histology of the foregut of Portunus sanguinolentus (Crustacea: Brachyura). Folia Morphol 37:364–372
Tziouveli V, Bastos-Gomez G, Bellwood O (2011) Functional morphology of mouthparts and digestive system during larval development of the cleaner shrimp Lysmata amboinensis (de Man, 1888). J Morphol 272:1080–1091
Vernon GM, Herold L, Witkus ER (1974) Fine structure of the digestive tract epithelium in the terrestrial isopod Armadillidium vulgare. J Morphol 144:337–359
Wägele J-W, Welsch U, Müller W (1981) Fine structure and function of the digestive tract of Cyathura carinata (Krøyer) (Crustacea, Isopoda). Zoomorphology 98:69–88
Watling L (2013) Feeding and digestive system. In: Watling L, Thiel M (eds) The Natural History of Crustacea, vol 1. Oxford University Press, Functional Morphology and Diversity, pp 237–260
Wigglesworth VB (1972) Digestion and nutrition. The Principles of Insect Physiology. Springer, Netherlands, Dordrecht, pp 476–552
Williams RL (1944) The pre-zoea stage of Porcellana platycheles (Pennant). Preliminary anatomical and histological notes. J R Microsc Soc 64:1–15
Wirkner CS, Richter S (2013) Circulatory system and respiration. Natural History of Crustacea 1:376–412
Witkus ER, Grillo RS, Smith WJ (1969) Microtubule bundles in the hindgut epithelium of the woodlouse Oniscus ascellus. J Ultrastruct Res 29:182–190
Woods WC (1918) The alimentary canal of the larva of Altica bimarginata Say (Coleoptera)*. Ann Entomol Soc Am 11:283–313
Yoshikoshi K (1975) On the structure and function of the alimentary canal of Tigriopus japonicus (Copepoda; Harpacticoida)-I Histological structure. Nippon Suisan Gakkaishi 41:929–935
Žnidaršič N, Mrak P, Tušek-Žnidarič M, Štrus J (2012) Exoskeleton anchoring to tendon cells and muscles in molting isopod crustaceans. ZooKeys 39
Acknowledgements
The authors would like to thank to the technicians at IRTA, Sant Carles de la Ràpita (David Carmona, Glòria Macià, Magda Monllaó, Francesc X. Ingla and Olga Bellot) and CCiTUB Hospital Clinic, Barcelona (Adriana Martínez, Almudena García, José Manuel Rebled and Rosa Rivera) for their assistance.
Funding
G.G. INIA Project (grant number RTA2011-00004-00-00) funded by Ministerio de Economía y Competitividad (Spanish Ministry of Economy and Competitiveness). D.C. FPI-INIA fellowship (INIA Project RTA2011-00004-00-00) funded by Ministerio de Economía y Competitividad (Spanish Ministry of Economy and Competitiveness).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castejón, D., Rotllant, G., Ribes, E. et al. Description of the larval and adult hindgut tract of the common spider crab Maja brachydactyla Balss, 1922 (Brachyura, Decapoda, Malacostraca). Cell Tissue Res 384, 703–720 (2021). https://doi.org/10.1007/s00441-021-03446-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-021-03446-3