Abstract
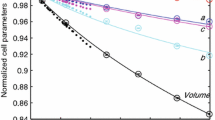
We collected in situ high-temperature powder X-ray diffraction (XRD) patterns, as well as Raman and Fourier transform infrared (FTIR) spectra on a natural serandite sample. The volumetric thermal expansion coefficient αV (K−1) is determined as a linear function of T (K): 37.6(5) × 10−9 × T + 11.1(3) × 10−6, with an averaged value of 31.7(10) × 10−6 K−1, while the anisotropy of axial thermal expansivities shows the order of αa > αb > αc. The isobaric Grüneisen parameters γiP are constrained as: 0.2–1.6 for most of the lattice vibrations below 500 cm−1; while 0–0.6 for the O–Si–O bending and Si–O stretching modes inside the Si3O9 chains above 500 cm−1. As compared with common silicate phases in the upper mantle and the transition zone, the γiP parameters for the internal vibrations are significantly smaller in silicate chains (serandite, enstatite) than those in Si2O7 groups (wadsleyite) and isolated SiO4 units (forsterite, pyrope), since the rotation of the bridging O atoms (in the direction perpendicular to Si–O–Si) could relax the SiO4 tetrahedra at elevated temperature. The OH-bending vibration of serandite is observed in both Raman-active (1378 cm−1) and IR-active (1389 cm−1) modes, with a temperature-dependence of − 0.07 and − 0.09 cm−1/K, respectively. The red-shift of these OH-bending modes with increasing temperature indicates that the hydrogen bond gets weaker as the O3…O4 distance becomes larger during thermal expansion. Additionally, our polarized FTIR spectra confirm that the stretching mode of the very strong hydrogen bond, a broad absorption region (1400–3300 cm−1), is polarized in the direction of E//b, as reported by Hammer et al. (Am Mineral 83:569–576, 1998).









Similar content being viewed by others
References
Arakcheeva A, Pattison P, Meisser N, Chapuis G, Pekov I, Thelin P (2007) New insight into the pectolite-serandite series: a single crystal diffraction study of Na(Ca1.73Mn0.27)HSi3O9 at 293 and 100 K. Z Kristallogr 222:696–704
Black L, Breen C, Yarwood J, Garbev K, Stemmermann P, Gasharova B (2007) Structural features of C-S-H(I) and its carbonation in Air-A Raman spectroscopic study. Part II: carbonated phases. J Am Ceram Soc 90:908–917
Cameron M, Papike JJ (1981) Structural and chemical variations in pyroxenes. Am Mineral 66:1–50
Cliffe MJ, Goodwin AL (2012) PASCal: a principal-axis strain calculator for thermal expansion and compressibility for thermal expansion and compressibility determination. J Appl Crystallogr 45:1321–1329
Dowty E (1987) Vibrational interactions of tetrahedra in silicate glasses and crystals I. calculations on ideal silicate-aluminate-germanate structural units. Phys Chem Miner 14:80–93
Du W, Clark SM, Walker D (2015) Thermo-compression of pyrope-grossular garnet solid solutions: non-linear compositional dependence. Am Mineral 100:215–222
Emsley J (1981) Very strong hydrogen bonding. Chem Soc Rev 9:91–124
Farmer VC (ed) (1974) The infrared spectra of minerals. Mineralogical Society, London
Frost RL, López A, Theiss FL, Romano AW, Scholz R (2015) A vibrational spectroscopic study of the silicate mineral pectolite—NaCa2Si3O8(OH). Spectrochim Acta A 134:58–62
Gillet P, Richet P, Guyot F, Figuet G (1991) High-temperature thermodynamic properties of forsterite. J Geophys Res 96:11805–11816
Gillet P, Fiquet G, Malezieux JM, Geiger CA (1992) High-pressure and high-temperature Raman spectroscopy of end-member garnets: pyrope, grossular and andradite. Eur J Mineral 4:651–664
Gillet P, Daniel I, Guyot F (1997) Anharmonic properties of Mg2SiO4-forsterite measured from the volume dependence of the Raman spectrum. Eur J Mineral 9:255–262
Hammer VMF, Libowitzky E, Rossman GR (1998) Single-crystal IR spectroscopy of very stong hydrogen bonds in pectolite, NaCa2[Si3O8(OH)], and serandite, NaCa2[Si3O8(OH)]. Am Mineral 83:569–576
Holl CM, Smyth JR, Jacobsen SD, Frost DJ (2008) Effects of hydration on the structure and compressibility of wadsleyite, β-(Mg2SiO4). Am Mineral 93:598–607
Holland TJB, Redfern SAT (1997) Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral Mag 61:65–77
Jackson JM, Palko JW, Andrault D, Sinogeikin SV, Lakshtanov DL, Wang J, Bass JD, Zha C-S (2003) Thermal expansion of natural orthoenstatite up to 1473 K. Eur J Mineral 97:6842–6866
Jacobsen SD, Smyth JR, Swope RJ, Sheldon RI (2000) Two proton positions in the very strong hydrogen bond of serandite, NaMn2[Si3O8(OH)]. Am Mineral 85:745–752
Kolesov BA, Geiger CA (2000) Low-temperature single-crystal Raman spectrum of pyrope. Phys Chem Miner 27:645–649
Kroll H, Kirfel A, Heinemann R, Barbier B (2012) Volume thermal expansion and related thermophysical parameters in the Mg, Fe olivine solid-solution series. Eur J Mineral 24:935–956
Libowitzky E (1999) Correlation of O–H stretching frequencies and O–H…O hydrogen bond lengths in minerals. Monatsh Chem 130:1047–1059
Lightfoot P, Woodcock DA, Maple MJ, Villaescusa LA, Wright PA (2001) The widespread of occurrence of negative thermal expansion in zeolites. J Mater Chem 11:212–216
Ohashi Y, Finger LW (1978) The role of octahedral cations in pyroxenoid crystal chemistry. I. bustamite, wollastonite, and the pectolite-schizolite-serandite series. Am Mineral 63:274–288
Okada T, Narita T, Nagai T, Yamanaka T (2008) Comparative Raman spectroscopic study on ilmenite-type MgSiO3 (akimotoite), MgGeO3, and MgTiO3 (geikielite) at high temperatures and high pressures. Am Mineral 93:39–47
Prewitt CT (1967) Refinement of the structure of pectolite, Ca2NaHSi3O9. Z Kristallogr 125:298–302
Prewitt CT, Buerger MJ (1963) Comparison of the crystal structures of wollanstonite and pectolite. Min Soc Am 1:293–302
Reynard B, Rubie DC (1996) High-pressure, high-temperature Raman spectroscopic study of ilmenite-type MgSiO3. Am Mineral 81:1092–1096
Reynard B, Takir F, Guyot F, Gwanmesia GD, Liebermann RC, Gillet P (1996) High-temperature Raman spectroscopic and X-ray diffraction study of β-Mg2SiO4: insights into its high- temperature thermodynamic porperties and the β- to α-phase-transformation mechanism and kinetics. Am Mineral 81:585–594
Takéuchi Y, Kudoh Y (1977) Hydrogen bonding and cation ordering in Magnet Cove pectolite. Z Kristallogr 146:281–292
Takéuchi Y, Kudoh Y, Haga N (1973) The interpretation of partial patterson functions and its application to structure analyses of serandite Mn2NaHSi3O9 and banalsite BaNa2Al4Si4O16. Z Kristallogr 138:313–336
Takéuchi Y, Kudoh Y, Yamanaka T (1976) Crystal chemistry of the serandite-pectolite series and related minerals. Am Mineral 61:229–237
Trots M, Kurnosov A, Ballaran TB, Frost DJ (2012) High-temperature structural behaviors of anhydrous wadsleyite and forsterite. Am Mineral 97:1582–1590
Williams ER, Weller MT (2014) A variable-temperature neutron diffraction study of serandite: a Mn-silicate framework with a very strong, two-proton site, hydrogen bond. Am Mineral 99:1755–1760
Yang X, Dubrovinsky L, Manthilake MAGM, Wei Q (2012) High-pressure and high-temperature Raman spectroscopic study of hydrous wadsleyite (β-Mg2SiO4). Phys Chem Miner 39:57–64
Yang Y, Wang Z, Smyth JR, Liu J, **a Q (2015) Water effects on the anharmonic properties of forsterite. Am Mineral 100:2185–2190
Ye Y, Smyth JR, Jacobsen SD, Panero WR, Brown DA, Katsura T, Chang Y-Y, Townsend JP, Dera P, Tkachev S, Unterborn C, Liu Z, Goujon C (2013) Crystal structure, Raman and FTIR spectroscopy, and equations of state of OH-bearing MgSiO3 akimotoite. Contrib Mineral Petrol 166:1375–1388
Ye Y, Jacobsen SD, Mao Z, Duffy TS, Hirner SM, Smyth JR (2015) Crystal structure, thermal expansivity, and elasticity of OH-chondrodite: trends among dense hydrous magnesium silicate. Contrib Mineral Petrol 169:43
Zucker R, Shim S-H (2009) In situ Raman spectroscopy of MgSiO3 enstatite up to 1550 K. Am Mineral 94:1638–1646
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant no. 2016YFC0600204), and the National Natural Science Foundation of China (Grant nos. 41590621 and 41672041). Raman spectra were measured at the center of Physics Experiment Teaching, University of Science and Technology of China, while XRD and FTIR measurements were conducted at the Micro-FTIR Laboratory in the Department of Earth Sciences, Institute of Geology and Geophysics, Zhejiang University. We acknowledge Dr. Zhilei Sui and ** Wang
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Ye, Y., Li, L., Smyth, J.R. et al. High-temperature X-ray diffraction, Raman and IR spectroscopy on serandite. Phys Chem Minerals 46, 705–715 (2019). https://doi.org/10.1007/s00269-019-01032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-019-01032-2