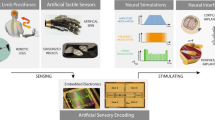
Abstract
Neuromodulation of the somatosensory nervous system is an effective tool to restore sensory feedback to individuals with sensory deficits, treat chronic pain, and study sensory processing pathways. This chapter presents an overview on neuromodulation techniques for somatosensation focused on clinical systems implemented in humans. The first section briefly reviews the key physiology of tactile and proprioceptive sensation. The second section describes different technologies and methods for evoking somatosensory percepts with electrical stimulation. The third section explains how to elicit and shape sensation produced by neurostimulation, drawing on key insights from recent research in human studies of neuroprostheses. This section is divided into the four perceptual dimensions of sensation: location, intensity, quality, and timing. We present the neural coding principles underlying each perceptual dimension in the normal sensory system, along with strategies to modulate each perceptual dimension with neural stimulation. The last section provides an overview of clinical applications of electrically evoked somatosensation, highlighted by an example in rehabilitation.
Similar content being viewed by others
Abbreviations
- APC:
-
Anterior parietal cortex
- DBS:
-
Deep brain stimulation
- DRG:
-
Dorsal root ganglion
- ECoG:
-
Electrocorticography
- FINE:
-
Flat interface nerve electrode
- fMRI:
-
Functional magnetic resonance imaging
- ICMS:
-
Intracortical microstimulation
- JND:
-
Just noticeable difference
- LIFE:
-
Longitudinal intrafascicular electrode
- LTMR:
-
Low-threshold mechanoreceptor
- MEG:
-
Magnetoencephalography
- PA:
-
Pulse amplitude
- PC:
-
Pacinian corpuscle (rapidly adapting type II)
- PF:
-
Pulse frequency
- PW:
-
Pulse width
- RA:
-
Rapidly adapting
- RAI:
-
Rapidly adapting type I
- RAII:
-
Rapidly adapting type II
- S1:
-
Primary somatosensory cortex
- SA:
-
Slowly adapting
- SAI:
-
Slowly adapting type I
- SAII:
-
Slowly adapting type II
- SCS:
-
Spinal cord stimulation
- TIME:
-
Transverse intrafascicular electrode
- USEA:
-
Utah slant microelectrode array
- Vc:
-
Ventrocaudal nucleus of the thalamus
References
Kandel, E.R., Schwartz, J.H., Jessell, T.M., et al.: Principles of Neural Science, 5th edn. McGraw-Hill Companies, Inc, New York City (2013)
Abraira, V.E., Ginty, D.D.: The sensory neurons of touch. Neuron. 79, 618–639 (2013). https://doi.org/10.1016/j.neuron.2013.07.051
Johansson, R.S., Flanagan, J.R.: Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci. 10, 345–359 (2009). https://doi.org/10.1038/nrn2621
Johansson, R.S., Vallbo, A.B.: Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 6, 27–32 (1983). https://doi.org/10.1016/0166-2236(83)90011-5
Proske, U., Gandevia, S.C.: The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697 (2012). https://doi.org/10.1152/physrev.00048.2011
Löken, L.S., Wessberg, J., Morrison, I., et al.: Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 12, 547–548 (2009). https://doi.org/10.1038/nn.2312
McGlone, F., Wessberg, J., Olausson, H.: Discriminative and affective touch: sensing and feeling. Neuron. 82, 737–755 (2014)
Delhaye, B.P., Long, K.H., Bensmaia, S.J.: Neural basis of touch and proprioception in primate cortex. Compr. Physiol. 8, 1575–1602 (2018). https://doi.org/10.1002/cphy.c170033
Grigg, P.: Peripheral neural mechanisms in proprioception. J. Sport Rehabil. 3, 2–17 (1994)
Gustafson, K.J., Pinault, G.C.J., Neville, J.J., et al.: Fascicular anatomy of human femoral nerve: implications for neural prostheses using nerve cuff electrodes. J. Rehabil. Res. Dev. 46, 973–984 (2009). https://doi.org/10.1682/JRRD.2008.08.0097
Brill, N.A., Tyler, D.J.: Quantification of human upper extremity nerves and fascicular anatomy. Muscle Nerve. 56, 463–471 (2017). https://doi.org/10.1002/mus.25534
Grinberg, Y., Schiefer, M.A., Tyler, D.J., Gustafson, K.J.: Fascicular perineurium thickness, size, and position affect model predictions of neural excitation. IEEE Trans. Neural Syst. Rehabil. Eng. 16, 572–581 (2008). https://doi.org/10.1109/TNSRE.2008.2010348
Haberberger, R.V., Barry, C., Dominguez, N., et al.: Human dorsal root ganglia. Front. Cell. Neurosci. 13, 1–17 (2019). https://doi.org/10.3389/fncel.2019.00271
Weiss, N., Ohara, S., Johnson, K.O., Lenz, F.A.: The human thalamic somatic sensory nucleus [Ventral caudal (Vc)] shows neuronal mechanoreceptor-like responses to optimal stimuli for peripheral mechanoreceptors. J. Neurophysiol. 101, 1033–1042 (2009). https://doi.org/10.1152/jn.90990.2008
Loutit, A.J., Vickery, R.M., Potas, J.R.: Functional organization and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub. J. Comp. Neurol., 1–34 (2020). https://doi.org/10.1002/cne.24942
Dijkerman, H.C., de Haan, E.H.F.: Somatosensory processes subserving perception and action. Behav. Brain Sci. 30, 189–201 (2007). https://doi.org/10.1017/S0140525X07001392
McFadyen, J., Dolan, R.J., Garrido, M.I.: The influence of subcortical shortcuts on disordered sensory and cognitive processing. Nat. Rev. Neurosci., 1–13 (2020). https://doi.org/10.1038/s41583-020-0287-1
Kandel, E.R.: From nerve cells to cognition: the internal representations of space and action. Princ. Neural Sci., 370–391 (2013)
Jones, L.A., Tan, H.Z.: Application of psychophysical techniques to haptic research. IEEE Trans. Haptics. 6, 268–284 (2013). https://doi.org/10.1109/TOH.2012.74
Gescheider, G.A.: Psychophysics: The Fundamentals, 3rd edn. Lawrence Erlbaum Associates, Inc, Mahway (1997)
Ortiz-Catalan, M., Brånemark, R., Håkansson, B., Delbeke, J.: On the viability of implantable electrodes for the natural control of artificial limbs: review and discussion. Biomed. Eng. Online. 11, 33 (2012). https://doi.org/10.1186/1475-925X-11-33
Günter, C., Delbeke, J., Ortiz-catalan, M.: Safety of long-term electrical peripheral nerve stimulation: review of the state of the art. 8, 1–16 (2019). https://doi.org/10.1186/s12984-018-0474-8
Forst, J.C., Blok, D.C., Slopsema, J.P., et al.: Surface electrical stimulation to evoke referred sensation. J. Rehabil. Res. Dev. 52, 397–406 (2015). https://doi.org/10.1682/JRRD.2014.05.0128
Perovic, M., Stevanovic, M., Jevtic, T., et al.: Electrical stimulation of the forearm: a method for transmitting sensory signals from the artificial hand to the brain. J. Autom. Control. 21, 13–18 (2013). https://doi.org/10.2298/JAC1301013P
Dietrich, C., Walter-Walsh, K., Preissler, S., et al.: Sensory feedback prosthesis reduces phantom limb pain: proof of a principle. Neurosci. Lett. 507, 97–100 (2012). https://doi.org/10.1016/j.neulet.2011.10.068
Geng, B., Jensen, W.: Human ability in identification of location and pulse number for electrocutaneous stimulation applied on the forearm. J. Neuroeng. Rehabil. 11, 97 (2014). https://doi.org/10.1186/1743-0003-11-97
Anani, A.B., Ikeda, K., Korner, L.M., Körner, L.M.: Human ability to discriminate various parameters in afferent electrical nerve stimulation with particular reference to prostheses sensory feedback. Med. Biol. Eng. Comput. 15, 363–373 (1977). https://doi.org/10.1007/Bf02457988
Prior, R.E., Lyman, J., Case, P.A., Scott, C.M.: Supplemental sensory feedback for the VA/NU myoelectric hand. Background and preliminary designs. Bull. Prosthet. Res. 101, 170–191 (1976)
Kaczmarek, K.A., Bach-y-Rita, P.: Tactile displays. In: Virtual Environments and Advanced Interface Design, pp. 349–414 (1995)
Kaczmarek, K.A., Webster, J.G., Bach-y-Rita, P., Tompkins, W.J.: Electrotactile and vibrotactile displays for sensory substitution systems. IEEE Trans. Biomed. Eng. 38, 1–16 (1991). https://doi.org/10.1109/10.68204
Naples, G.G., Mortimer, J.T., Scheiner, A., Sweeney, J.D.: A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans. Biomed. Eng. 35, 905–916 (1988). https://doi.org/10.1109/10.8670
Veraart, C., Grill, W.M., Mortimer, J.T.: Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans. Biomed. Eng. 40, 640–653 (1993). https://doi.org/10.1109/10.237694
Tyler, D.J., Durand, D.M.: Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 10, 294–303 (2002). https://doi.org/10.1109/TNSRE.2002.806840
Tyler, D.J., Durand, D.M.: Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Ann. Biomed. Eng. 31, 633–642 (2003). https://doi.org/10.1114/1.1569263
Tan, D.W., Schiefer, M.A., Keith, M.W., et al.: A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 6, 1–12 (2014). https://doi.org/10.1126/scitranslmed.3008669
Freeberg, M.J., Stone, M.A., Triolo, R.J., Tyler, D.J.: The design of and chronic tissue response to a composite nerve electrode with patterned stiffness. J. Neural Eng. 14, 036022 (2017). https://doi.org/10.1088/1741-2552/aa6632
Christie, B.P., Freeberg, M., Memberg, W.D., et al.: Long-term stability of stimulating spiral nerve cuff electrodes on human peripheral nerves. J. Neuroeng. Rehabil. 14, 1–12 (2017). https://doi.org/10.1186/s12984-017-0285-3
Yoshida, K., Pellinen, D., Pivin, D., et al.: Development of the thin-film longitudinal intra-fascicular electrode. Proc. Fifth Annu. Conf. IFESS, 3–5 (2000)
Yoshida, K., Hennings, K., Kammer, S.: Acute performance of the thin-film longitudinal intra-fascicular electrode. First IEEE/RAS-EMBS Int Conf Biomed Robot Biomechatronics. BioRob. 2006, 296–300 (2006). https://doi.org/10.1109/BIOROB.2006.1639102
Lawrence, S.M., Dhillon, G.S., Horch, K.W.: Fabrication and characteristics of an implantable, polymer-based, intrafascicular electrode. J. Neurosci. Methods. 131, 9–26 (2003). https://doi.org/10.1016/S0165-0270(03)00231-0
Dhillon, G.S., Lawrence, S.M., Hutchinson, D.T., Horch, K.W.: Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J. Hand Surg. Am. 29, 605–615; discussion 616–8 (2004). https://doi.org/10.1016/j.jhsa.2004.02.006
Lawrence, S.M., Larsen, J.O., Horch, K.W., et al.: Long-term biocompatibility of implanted polymer-based intrafascicular electrodes. J. Biomed. Mater. Res. An Off. J. Soc. Biomater. Japanese Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 63, 501–506 (2002). https://doi.org/10.1002/jbm.10303
Boretius, T., Badia, J., Pascual-Font, A., et al.: A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 26, 62–69 (2010). https://doi.org/10.1016/j.bios.2010.05.010
Raspopovic, S., Capogrosso, M., Petrini, F.M., et al.: Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 6, 222ra19 (2014). https://doi.org/10.1126/scitranslmed.3006820
Normann, R.A., McDonnall, D., Clark, G.A., et al.: Physiological activation of the hind limb muscles of the anesthetized cat using the utah slanted electrode array. Proc. Int. Jt. Conf. Neural Networks. 5, 3103–3108 (2005). https://doi.org/10.1109/IJCNN.2005.1556422
Branner, A., Stein, R.B., Fernandez, E., et al.: Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed. Eng. 51, 146–157 (2004). https://doi.org/10.1109/TBME.2003.820321
Branner, A., Stein, R.B., Normann, R.A.: Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J. Neurophysiol. 85, 1585–1594 (2001)
Clark, G.A., Wendelken, S., Page, D.M., et al.: Using multiple high-count electrode arrays in human median and ulnar nerves to restore sensorimotor function after previous transradial amputation of the hand. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 1977–1980 (2014). https://doi.org/10.1109/EMBC.2014.6944001
Tyler, D.J., Durand, D.M.: A slowly penetrating interfascicular nerve electrode for selective activation of peripheral nerves. IEEE Trans. Rehabil. Eng. 5, 51–61 (1997)
Liem, L., Russo, M., Huygen, F.J.P.M., et al.: A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain, 471–482 (2013). https://doi.org/10.1111/ner.12072
Lynch, P.J., Mcjunkin, T., Eross, E., et al.: Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root ganglion for postherpetic neuralgia. 2010, 58–61 (2011). https://doi.org/10.1111/j.1525-1403.2010.00307.x
Deer, T.R., Levy, R.M., Kramer, J., et al.: Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 158, 669 (2017)
Weiner, R.L., Yeung, A., Garcia, C.M., et al.: Treatment of FBSS low back pain with a novel percutaneous DRG wireless stimulator: pilot and feasibility study. Pain Med. 17, 1911–1916 (2016). https://doi.org/10.1093/pm/pnw075
Nanivadekar, A.C., Ayers, C.A., Gaunt, R.A., et al.: Selectivity of afferent microstimulation at the DRG using epineural and penetrating electrode arrays. J. Neural Eng. 17 (2020)
Ayers, C.A., Fisher, L.E., Gaunt, R.A., Weber, D.J.: Microstimulation of the lumbar DRG recruits primary afferent neurons in localized regions of lower limb. J. Neurophysiol. 116, 51–60 (2016). https://doi.org/10.1152/jn.00961.2015
Fisher, L.E., Ayers, C.A., Ciollaro, M.: Chronic recruitment of primary afferent neurons by microstimulation in the feline dorsal root ganglia. (2014). https://doi.org/10.1088/1741-2560/11/3/036007
Chandrasekaran, S., Nanivadekar, A.C., McKernan, G., et al.: Sensory restoration by epidural stimulation of the lateral spinal cord in upper-limb amputees. elife. 9, 1–26 (2020). https://doi.org/10.7554/eLife.54349
Grider, J., Manchikanti, L., Carayannopoulos, A., et al.: Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician. 19, 33–54 (2016)
North, R.B., Kidd, D.H., Petrucci, L., Dorsi, M.J.: Spinal cord stimulation electrode design: a prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: Part II – Clinical outcomes. Neurosurgery. 57, 990–995 (2005). https://doi.org/10.1227/01.NEU.0000180030.00167.b9
Villavicencio, A.T., Leveque, J.C., Rubin, L., et al.: Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 46, 399–406 (2000). https://doi.org/10.1097/00006123-200002000-00025
Epstein, L.J., Palmieri, M.: Managing chronic pain with spinal cord stimulation. Mt. Sinai J. Med. 79, 123–132 (2012). https://doi.org/10.1002/MSJ
Kiss, Z.H.T., Anderson, T., Hansen, T., et al.: Neural substrates of microstimulation-evoked tingling: a chronaxie study in human somatosensory thalamus. Eur. J. Neurosci. 18, 728–732 (2003). https://doi.org/10.1046/j.1460-9568.2003.02793.x
Heming, E., Sanden, A., Kiss, Z.: Designing a somatosensory neural prosthesis: percepts evoked by different patterns of thalamic stimulation. J. Neural Eng. 064001 (2010). https://doi.org/10.1088/1741-2560/7/6/064001
Heming, E., Choo, R.: Designing a thalamic somatosensory neural prosthesis: consistency and persistence of percepts evoked by electrical stimulation. Neural Syst …. 19, 477–482 (2011)
Tasker, R.R., Kiss, Z.H.T.: The role of the thalamus in functional neurosurgery. Neurosurg. Clin. N. Am. 6, 73–104 (1995)
Hiremath, S.V., Tyler-Kabara, E.C., Wheeler, J.J., et al.: Human perception of electrical stimulation on the surface of somatosensory cortex. PLoS One. 12, 1–16 (2017). https://doi.org/10.1371/journal.pone.0176020
Lee, B., Kramer, D., Salas, M.A., et al.: Engineering artificial somatosensation through cortical stimulation in humans. 12, 1–11 (2018). https://doi.org/10.3389/fnsys.2018.00024
Flesher, S.N., Collinger, J.L., Foldes, S.T., et al.: Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 8, 1–11 (2016). https://doi.org/10.1126/scitranslmed.aaf8083
Armenta Salas, M., Bashford, L., Kellis, S., et al.: Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. elife. 7, 1–11 (2018). https://doi.org/10.7554/eLife.32904
Fifer, M.S., McMullen, D.P., Thomas, T.M., et al.: Intracortical microstimulation elicits human fingertip sensations. medRxiv, 2020.05.29.20117374 (2020). https://doi.org/10.1101/2020.05.29.20117374
Nowak, L.G., Bullier, J.: Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. Exp. Brain Res. 118, 477–488 (1998)
Richardson, A.G., McIntyre, C.C., Grill, W.M.: Modelling the effects of electric fields on nerve fibres: influence of the myelin sheath. Med. Biol. Eng. Comput. 38, 438–446 (2000). https://doi.org/10.1007/BF02345014
Histed, M.H., Ni, A.M., Maunsell, J.H.R.: Insights into cortical mechanisms of behavior from microstimulation experiments. Prog. Neurobiol. 103, 115–130 (2013). https://doi.org/10.1016/j.pneurobio.2012.01.006
McIntyre, C., Grill, W.M.: Selective microstimulation of central nervous system neurons. Ann. Biomed. Eng. 28, 219–233 (2000)
Schiefer, M.A., Tyler, D.J.: Computer models of peripheral nerves. In: Nerves and Nerve Injuries: Volume 2: Pain, Treatment, Injury, Disease, and Future Directions, 1st edn, pp. 1–993. Elsevier Inc. (2015)
Peckham, P.H., Knutson, J.S.: Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 7, 327–360 (2005). https://doi.org/10.1146/annurev.bioeng.6.040803.140103
Gorman, P.H., Mortimer, J.T.: The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Trans. Biomed. Eng. BME. 30, 407–414 (1983). https://doi.org/10.1109/TBME.1983.325041
Mogyoros, I., Kiernan, M.C., Burke, D.: Strength-duration properties of human peripheral nerve. Brain. 119, 439–447 (1996). https://doi.org/10.1093/brain/119.2.439
Gooch, C.L., Doherty, T.J., Chan, K.M., et al.: Motor unit number estimation: a technology and literature review. Muscle Nerve. 50, 884–893 (2014). https://doi.org/10.1002/mus.24442
Corniani, G., Saal, H.P.: Tactile innervation densities across the whole body. J. Neurophysiol. 124(4), 1229–1240 (2020)
Gaines, J., Finn, K., Slopsema, J., et al.: A model of median nerve activation using surface electrical stimulation. J. Comput. Neurosci. 45, 29–43 (2018). https://doi.org/10.1007/s10827-018-0689-5
Rose, T.L., Robblee, L.S.: Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses. IEEE Trans. Biomed. Eng. 37, 1118–1120 (1990). https://doi.org/10.1109/10.61038
Shannon, R.V.: A model of safe levels for electrical stimulation. IEEE Trans. Biomed. Eng. 39, 424–426 (1992). https://doi.org/10.1109/10.126616
McCreery, D.B., Agnew, W.F., Yuen, T.G.H., Bullara, L.: Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 37, 996–1001 (1990)
Merrill, D.R., Bikson, M., Jefferys, J.G.R.: Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods. 141, 171–198 (2005). https://doi.org/10.1016/j.jneumeth.2004.10.020
Ochoa, J., Torebjork, E.: Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J. Physiol. 342, 633–654 (1983)
Van Den Honert, C., Mortimer, J.T.: A technique for collision block of peripheral nerve: single stimulus analysis. IEEE Trans. Biomed. Eng. 28, 373–378 (1981). https://doi.org/10.1109/TBME.1981.324718
Van Den Honert, C., Mortimer, J.T.: A technique for collision block of peripheral nerve: frequency dependence. IEEE Trans. Biomed. Eng. 28, 379–382 (1981). https://doi.org/10.1109/TBME.1981.324719
Histed, M.H., Bonin, V., Reid, R.C.: Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 63, 508–522 (2009). https://doi.org/10.1016/j.neuron.2009.07.016
Rutten, W.L.C., Van Wier, H.J., Put, J.H.M.: Sensitivity and selectivity of intraneural stimulation using a silicon electrode array. IEEE Trans. Biomed. Eng. 38, 192–198 (1991). https://doi.org/10.1109/10.76386
Grill, W.M., Mortimer, J.T.: The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans. Biomed. Eng. 43, 161–166 (1996)
Grill, W.M., Mortimer, J.T.: Non-invasive measurement of the input-output properties of peripheral nerve stimulating electrodes. J. Neurosci. Methods. 65, 43–50 (1996). https://doi.org/10.1016/0165-0270(95)00143-3
Yoshida, K., Horch, K.: Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE Trans. Biomed. Eng. 40, 492–494 (1993). https://doi.org/10.1109/10.243412
Ekedahl, R., Frank, O., Hallin, R.G.: Peripheral afferents with common function cluster in the median nerve and somatotopically innervate the human palm. Brain Res. Bull. 42, 367–376 (1997)
Tyler, D.J., Peterson, E.J., Brill, N., White, K.: Increased selectivity of clinical peripheral nerve interfaces. 2011 5th Int. IEEE/EMBS Conf. Neural Eng. NER 2011, 257–260 (2011). https://doi.org/10.1109/NER.2011.5910536
Brill, N., Tyler, D.: Optimizing nerve cuff stimulation of targeted regions through use of genetic algorithms. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 5811–5814 (2011). https://doi.org/10.1109/IEMBS.2011.6091438
Manola, L., Holsheimer, J.: Technical performance of percutaneous and laminectomy leads analyzed by modeling. Neuromodulation. 7, 231–241 (2004). https://doi.org/10.1111/j.1094-7159.2004.04207.x
Vega-Bermudez, F., Johnson, K.O.: SA1 and RA receptive fields, response variability, and population responses mapped with a probe array. J. Neurophysiol. 81, 2701–2710 (1999). https://doi.org/10.1152/jn.1999.81.6.2701
Johansson, R.S., Vallbo, A.B.: Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 286, 283–300 (1979). https://doi.org/10.1113/jphysiol.1979.sp012619
Brown, P.B., Koerber, H.R., Millecchia, R.: From innervation density to tactile acuity: 1. Spatial representation. Brain Res. 1011, 14–32 (2004). https://doi.org/10.1016/j.brainres.2004.03.009
Mancini, F., Bauleo, A., Cole, J., et al.: Whole-body map** of spatial acuity for pain and touch. Ann. Neurol. 75, 917–924 (2014). https://doi.org/10.1002/ana.24179
Novak, C.B., Kelly, L., Mackinnon, S.E.: Sensory recovery after median nerve grafting. J. Hand Surg. Am. 17, 59–68 (1992). https://doi.org/10.1016/0363-5023(92)90114-5
Pasluosta, C., Kiele, P., Stieglitz, T.: Paradigms for restoration of somatosensory feedback via stimulation of the peripheral nervous system. Clin. Neurophysiol. (2017). https://doi.org/10.1016/j.clinph.2017.12.027
Serino, A., Haggard, P.: Touch and the body. Neurosci Bio. 34, 224–236 (2010). https://doi.org/10.1016/j.neubiorev.2009.04.004
Sur, M., Merzenich, M.M., Kaas, J.H.: Magnification, receptive-field area, and “hypercolumn” size in areas 3b and 1 of somatosensory cortex in owl monkeys. J. Neurophysiol. 44, 295–311 (1980). https://doi.org/10.1152/jn.1980.44.2.295
Sunderland, S.: The intraneural topography of the radial, median and ulnar nerves. Brain. 68, 243–298 (1945)
Jabaley, M.E., Wallace, W.H., Heckler, F.R.: Internal topography of major nerves of the forearm and hand: a current view. J. Hand Surg. Am. 5, 1–18 (1980). https://doi.org/10.1097/00006534-198011000-00072
Watchmaker, G.P., Gumucio, C.A., Crandall, R.E., et al.: Fascicular topography of the median nerve: a computer based study to identify branching patterns. J. Hand Surg. Am. 16, 53–59 (1991). https://doi.org/10.1016/S0363-5023(10)80013-9
Purves, D., Augustine, G.J., Fitzpatrick, D., et al.: Neuroscience. (2004)
Gaunt, R.A., Hokanson, J.A., Weber, D.J.: Microstimulation of primary afferent neurons in the L7 dorsal root ganglia using multielectrode arrays in anesthetized cats: thresholds and recruitment properties. J. Neural Eng. 6 (2009). https://doi.org/10.1088/1741-2560/6/5/055009
Wessels, W.J.T., Feirabend, H.K.P., Marani, E.: Evidence for a rostrocaudal organization in dorsal root ganglia during development as demonstrated by intra-uterine W G A – H R P injections into the hindlimb of rat fetuses. 54, 273–281 (1990)
Prats-Galino, A., Puigdellívol-Sánchez, A., Ruano-Gil, D., Molander, C.: Representations of hindlimb digits in rat dorsal root ganglia. J. Comp. Neurol. 408, 137–145 (1999). https://doi.org/10.1002/(SICI)1096-9861(19990524)408:1<137::AID-CNE10>3.0.CO;2-3
Aoyagi, Y., Stein, R.B., Branner, A., et al.: Capabilities of a penetrating microelectrode array for recording single units in dorsal root ganglia of the cat. J. Neurosci. Methods. 128, 9–20 (2003). https://doi.org/10.1016/S0165-0270(03)00143-2
Wessels, W.J.T., Feirabend, H.K.P., Marani, E.: The rostrocaudal organization in the dorsal root ganglia of the rat: a consequence of plexus formation? Anat Embryol (Berl). 190, 1–11 (1994). https://doi.org/10.1007/BF00185841
Werner, G., Whitsel, B.L.: The topology of dermatomal projection in the medial lemniscal system. J. Physiol. 192, 123–144 (1967). https://doi.org/10.1113/jphysiol.1967.sp008292
Smith, M.C., Deacon, P.: Topographical anatomy of the posterior columns of the spinal cord in man: the long ascending fibres. Brain. 107, 671–698 (1984). https://doi.org/10.1093/brain/107.3.671
Gardner, E., Martin, J.: Coding of sensory information. In: Principles of Neural Science, pp. 421–429 (2000)
Blankenburg, F., Ruben, J., Meyer, R., et al.: Evidence for a rostral-to-caudal somatotopic organization in human primary somatosensory cortex with mirror-reversal in areas 3b and 1. Cereb. Cortex. 13, 987–993 (2003). https://doi.org/10.1093/cercor/13.9.987
Iwamura, Y., Iriki, A., Tanaka, M.: Bilateral hand representation in the postcentral somatosensory cortex. Nature. 369, 554–556 (1994). https://doi.org/10.1038/369554a0
Iwamura, Y., Tanaka, M., Sakamoto, M., Hikosaka, O.: Converging patterns of finger representation and complex response properties of neurons in area 1 of the first somatosensory cortex of the conscious monkey. Exp. Brain Res. 51, 327–337 (1983). https://doi.org/10.1007/BF00237869
Petrini, F.M., Valle, G., Bumbasirevic, M., et al.: Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 11, 8939 (2019)
Charkhkar, H., Shell, C.E., Marasco, P.D., et al.: High-density peripheral nerve cuffs restore natural sensation to individuals with lower-limb amputations. J. Neural Eng. 15, 056002 (2018). https://doi.org/10.1088/1741-2552/aac964
Tan, D.W., Schiefer, M.A., Keith, M.W., et al.: Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in a human amputee. J. Neural Eng. 12, 859–862 (2015). https://doi.org/10.1109/NER.2013.6696070
Clark, G.A., Wendelken, S., Page, D.M., et al.: Using multiple high-count electrode arrays in human median and ulnar nerves to restore sensorimotor function after previous transradial amputation of the hand. 2014 36th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBC. 2014, 1977–1980 (2014). https://doi.org/10.1109/EMBC.2014.6944001
Dhillon, G.S., Krüger, T.B., Sandhu, J.S., et al.: Effects of short-term training on sensory and motor function in severed nerves of long-term human amputees. J. Neurophysiol. 93, 2625–2633 (2005). https://doi.org/10.1152/jn.00937.2004
Muniak, M.A., Ray, S., Hsiao, S.S., et al.: The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J. Neurosci. 27, 11687–11699 (2007). https://doi.org/10.1523/JNEUROSCI.1486-07.2007
Bensmaia, S.J.: Tactile intensity and population codes. Behav. Brain Res. 190, 165–173 (2008). https://doi.org/10.1016/j.bbr.2008.02.044
Graczyk, E.L., Schiefer, M.A., Saal, H.P., et al.: The neural basis of perceived intensity in natural and artificial touch. Sci. Transl. Med. 8, 1–11 (2016). https://doi.org/10.1126/scitranslmed.aaf5187
Gescheider, G.A.: Psychophysical measurement of thresholds. In: Psychophysics: Method, Theory, and Application, pp. 1–37 (1985)
Manfredi, L.R., Baker, A.T., Elias, D.O., et al.: The effect of surface wave propagation on neural responses to vibration in primate glabrous skin. PLoS One. 7, e31203 (2012). https://doi.org/10.1371/journal.pone.0031203
Johnson, K.O.: Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J. Neurophysiol. 37, 48–72 (1974)
Talbot, W.H., Darian-Smith, I., Kornhuber, H.H., Mountcastle, V.B.: The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J. Neurophysiol. 31, 301–334 (1968)
Freeman, B.Y.A.W., Johnson, K.: A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkeys. J. Physiol. 323, 43–64 (1982)
McGlone, F., Reilly, D.: The cutaneous sensory system. Neurosci. Biobehav. Rev. 34, 148–159 (2010). https://doi.org/10.1016/j.neubiorev.2009.08.004
Mountcastle, V.B., Talbot, W.H., Sakata, H., Hyvärinen, J.: Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J. Neurophysiol. 32, 452–484 (1969). https://doi.org/10.1152/jn.1969.32.3.452
Knibestöl, M., Vallbo, B., Knibestol, B.Y.M., Vallbo, A.B.: Intensity of sensation related to activity of slowly adapting mechanoreceptive units in the human hand. J. Physiol. 300, 251–267 (1980). https://doi.org/10.1113/jphysiol.1980.sp013160
Leung, Y.Y., Bensmaia, S.J., Hsiao, S.S., Johnson, K.O.: Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents. J. Neurophysiol. 94, 3037–3045 (2005). https://doi.org/10.1152/jn.00002.2005
Verrillo, R.T., Gescheider, G.A.: Effect of prior stimulation on vibrotactile thresholds. Sens. Processes. 1, 292–300 (1977)
Gescheider, G.A., Wright, J.H.: Effects of sensory adaptation on the form of the psychophysical magnitude function for cutaneous vibration. J. Exp. Psychol. 77, 308–313 (1968). https://doi.org/10.1037/h0025746
Hollins, M., Goble, A.K., Whitsel, B.L., Tommerdahl, M.: Time course and action spectrum of vibrotactile adaptation. Somatosens. Mot. Res. 7, 205–221 (1990). https://doi.org/10.3109/08990229009144707
Bensmaïa, S.J., Leung, Y.Y., Hsiao, S.S., Johnson, K.O.: Vibratory adaptation of cutaneous mechanoreceptive afferents. J. Neurophysiol. 94, 3023–3036 (2005). https://doi.org/10.1152/jn.00002.2005
Berglund, U., Berglund, B.: Adaptation and recovery in vibrotactile perception. Percept. Mot. Skills. 30, 843–853 (1970)
Hahn, J.F.: Vibrotactile adaptation and recovery measured by two methods. J. Exp. Psychol. 71, 655–658 (1966). https://doi.org/10.1037/h0023094
Brenner, N., Bialek, W., de van Steveninck, R.R.: Adaptive rescaling maximizes information transmission. Neuron. 26, 695–702 (2000)
Ribot-Ciscar, E., Roll, J.P., Tardy-Gervet, M.F., Harlay, F.: Alteration of human cutaneous afferent discharges as the result of long-lasting vibration. J. Appl. Physiol. 80, 1708–1715 (1996)
Whitsel, B.L., Kelly, E.F., Quibrera, M., et al.: Time-dependence of SI RA neuron response to cutaneous flutter stimulation. Somatosens. Mot. Res. 20, 45–69 (2003). https://doi.org/10.1080/0899022031000083834
O’Mara, S., Rowe, M.J., Tarvin, R.P.: Neural mechanisms in vibrotactile adaptation. J. Neurophysiol. 59, 607–622 (1988)
Bostock, B.Y.H., Sears, T.A., Sherratt, R.M.: Spatial distribution of excitability. J. Physiol. 34, 41–58 (1983)
Vallbo, A.B., Johansson, R.S.: Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum. Neurobiol. 3, 3–14 (1984)
Menia, L.L., van Doren, C.L.: Independence of pitch and loudness of an electrocutaneous stimulus for sensory feedback. IEEE Trans. Rehabil. Eng. 2, 197–206 (1994)
Szeto, A.Y.J., Lyman, J., Prior, R.E.: Electrocutaneous pulse rate and pulse width psychometric functions for sensory communications. Hum. Factors J. Hum. Factors Ergon. Soc. 21, 241–249 (1979). https://doi.org/10.1177/001872087902100212
Szeto, A.Y.: Relationship between pulse rate and pulse width for a constant-intensity level of electrocutaneous stimulation. Ann. Biomed. Eng. 13, 373–383 (1985). https://doi.org/10.1007/BF02407767
Szeto, A.Y.J., Saunders, F.A.: Electrocutaneous stimulation for sensory communication in rehabilitation engineering. IEEE Trans. Biomed. Eng. 29, 300–308 (1982). https://doi.org/10.1109/TBME.1982.324948
Flesher, S.N., Downey, J.E., Weiss, J.M., et al.: Restored tactile sensation improves neuroprosthetic arm control. bioRxiv, 653428 (2019). https://doi.org/10.1101/653428
Hughes, C., Flesher, S., Weiss, J., et al.: Perceptual responses to microstimulation frequency are spatially organized in human somatosensory cortex. bioarxiv. (2020). https://doi.org/10.1101/2020.07.16.207506
Graczyk, E.L., Delhaye, B.P., Schiefer, M.A., et al.: Sensory adaptation to electrical stimulation of the somatosensory nerves. J. Neural Eng. 15 (2018). https://doi.org/10.1088/1741-2552/aab790
Buma, D.G., Buitenweg, J.R., Veltink, P.H.: Intermittent stimulation delays adaptation to electrocutaneous sensory feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 15, 435–441 (2007). https://doi.org/10.1109/TNSRE.2007.903942
Kaczmarek, K.A.: Electrotactile adaptation on the abdomen: preliminary results. IEEE Trans. Rehabil. Eng. 8, 499–505 (2000)
McCreery, D.B., Yuen, T.G.H., Agnew, W.F., Bullara, L.A.: A characterization of the effects on neuronal excitability due to prolonged microstimulation with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 44, 931–939 (1997). https://doi.org/10.1109/10.634645
Kim, S., Callier, T., Bensmaia, S.J.: A computational model that predicts behavioral sensitivity to intracortical microstimulation. J. Neural Eng. 14 (2017)
Anani, A.B., Körner, L.M.: Afferent electrical nerve stimulation: human tracking performance relevant to prosthesis sensory feedback. Med. Biol. Eng. 17, 425–434 (1979)
Hollins, M., Faldowski, R., Rao, S., Young, F.: Perceptual dimensions of tactile surface texture: a multidimensional scaling analysis. Percept. Psychophys. 54, 697–705 (1993)
Hollins, M., Bensmaïa, S., Karlof, K., Young, F.: Individual differences in perceptual space for tactile textures: evidence from multidimensional scaling. Percept. Psychophys. 62, 1534–1544 (2000)
Picard, D., Dacremont, C., Valentin, D., Giboreau, A.: Perceptual dimensions of tactile textures. Acta Psychol. 114, 165–184 (2003). https://doi.org/10.1016/j.actpsy.2003.08.001
Bergmann Tiest, W.M., Kappers, A.M.L.: Analysis of haptic perception of materials by multidimensional scaling and physical measurements of roughness and compressibility. Acta Psychol. 121, 1–20 (2006). https://doi.org/10.1016/j.actpsy.2005.04.005
Guest, S., Dessirier, J.M., Mehrabyan, A., et al.: The development and validation of sensory and emotional scales of touch perception. Atten. Percept. Psychophys. 73, 531–550 (2011). https://doi.org/10.3758/s13414-010-0037-y
Saal, H.P., Bensmaia, S.J.: Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci. 37, 689–697 (2014)
Schady, W.J., Torebjörk, H.E., Ochoa, J.L.: Peripheral projections of nerve fibres in the human median nerve. Brain Res. 277, 249–261 (1983)
Wu, G., Ekedahl, R., Stark, B., et al.: Clustering of Pacinian corpuscle afferent fibres in the human median nerve. Exp. Brain Res. 126, 399–409 (1999)
Wu, G., Ekedahl, R., Hallin, R.G.: Clustering of slowly adapting type II mechanoreceptors in human peripheral nerve and skin. Brain. 121(Pt 2), 265–279 (1998)
Roberts, W.J., Elardo, S.M.: Clustering of primary afferent fibers in peripheral nerve fascicles by sensory modality. Brain Res. 370, 149–152 (1986). https://doi.org/10.1016/0006-8993(86)91115-7
Niu, J., Ding, L., Li, J.J., et al.: Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J. Neurosci. 33, 17691–17709 (2013). https://doi.org/10.1523/JNEUROSCI.3429-13.2013
Lieber, J.D., Bensmaia, S.J.: High-dimensional representation of texture in somatosensory cortex of primates. Proc. Natl. Acad. Sci. 116 (2019). https://doi.org/10.1073/pnas.1818501116
Pei, Y.-C., Denchev, P.V., Hsiao, S.S., et al.: Convergence of submodality-specific input onto neurons in primary somatosensory cortex. J. Neurophysiol. 102, 1843–1853 (2009). https://doi.org/10.1152/jn.00235.2009
Mountcastle, V.B.: Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J. Neurophysiol. 20, 408–434 (1957)
Mountcastle, V.B.: The columnar organization of the neocortex. Brain: A J. Neurol. 120, 701–722 (1997)
Weber, A.I., Saal, H.P., Lieber, J.D., et al.: Spatial and temporal codes mediate the tactile perception of natural textures. Proc. Natl. Acad. Sci. 110, 17107–17112 (2013). https://doi.org/10.1073/pnas.1305509110
Tanaka, Y., Bergmann Tiest, W.M., Kappers, A.M.L., Sano, A.: Contact force and scanning velocity during active roughness perception. PLoS One. 9 (2014). https://doi.org/10.1371/journal.pone.0093363
Manfredi, L.R., Saal, H.P., Brown, K.J., et al.: Natural scenes in tactile texture. J. Neurophysiol. 111, 1792–1802 (2014). https://doi.org/10.1152/jn.00680.2013
Manfredi, L.R.: The role of vibratory cues in affective responses to tactile textures. Sch Mech Eng. (2010)
Graczyk, E.L., Christie, B.P., He, Q., et al.: Frequency shapes the quality of tactile percepts evoked through electrical stimulation of the nerves. bioRxiv. (2020)
Kim, L.H., Mcleod, R.S., Kiss, Z.H.T.: A new psychometric questionnaire for reporting of somatosensory percepts. J. Neural Eng. 15 (2018)
Valle, G., Iberite, F., Strauss, I., et al.: A psychometric platform to collect somatosensory sensations for neuroprosthetic use Equally author contributors † Corresponding authors. bioRxiv, 2020.07.23.218222 (2020). https://doi.org/10.1101/2020.07.23.218222
Geng, B., Yoshida, K., Petrini, L., Jensen, W.: Evaluation of sensation evoked by electrocutaneous stimulation on forearm in nondisabled subjects. J. Rehabil. Res. Dev. 49, 297–308 (2012)
Davis, T.S., Wark, H.A.C.C., Hutchinson, D.T., et al.: Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 13, 036001 (2016). https://doi.org/10.1088/1741-2560/13/3/036001
Clip**er, F.F.W., Avery, R., Titus, B.B.R.: A sensory feedback system for an upper-limb amputation prosthesis. Bull. Prosthet. Res., 247–258 (1974)
Graczyk, E.L., Resnik, L., Schiefer, M.A., et al.: Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 8, 9866 (2018). https://doi.org/10.1038/s41598-018-26952-x
Cuberovic, I., Gill, A., Resnik, L.J., et al.: Learning of artificial sensation through long-term home use of a sensory-enabled prosthesis. Front. Neurosci. 13, 1–24 (2019). https://doi.org/10.3389/fnins.2019.00853
Saal, H.P., Delhaye, B.P., Rayhaun, B.C., Bensmaia, S.J.: Simulating tactile signals from the whole hand with millisecond precision. Proc. Natl. Acad. Sci. 114, E5693–E5702 (2017). https://doi.org/10.1073/pnas.1704856114
Formento, E., D’anna, E., Gribi, S., et al.: A biomimetic electrical stimulation strategy to induce asynchronous stochastic neural activity. BioRxiv. (2019)
Okorokova, E., He, Q., Bensmaia, S.: Biomimetic encoding model for restoring touch in bionic hands through a nerve interface. J. Neural Eng. 15 (2018) 10pp
Valle, G., Mazzoni, A., Iberite, F., et al.: Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron. 100, 1–9 (2018). https://doi.org/10.1016/j.neuron.2018.08.033
Graczyk, E.L.: Natural Perceptual Characteristics and Psychosocial Impacts of Touch Evoked by Peripheral Nerve Stimulation. Case Western Reserve University (2018)
George, J.A., Kluger, D.T., Davis, T.S., et al.: Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci Robot. 4, 1–12 (2019). https://doi.org/10.1126/scirobotics.aax2352
Whitsel, B.L., Kelly, E.F., Delemos, K.A., et al.: Stability of rapidly adapting afferent entrainment vs responsivity. Somatosens. Mot. Res. 17, 13–31 (2000). https://doi.org/10.1080/08990220070265
Pongrac, H.: Vibrotactile perception: examining the coding of vibrations and the just noticeable difference under various conditions. Multimed Syst. 13, 297–307 (2007). https://doi.org/10.1007/s00530-007-0105-x
Mountcastle, V.B., Steinmetz, M.A., Romo, R.: Frequency discrimination in the sense of flutter: psychophysical measurements correlated with postcentral events in behaving monkeys. J. Neurosci. 10, 3032–3044 (1990). https://doi.org/10.1523/JNEUROSCI.10-09-03032.1990
Hursh, J.B.: Conduction velocity and diameter of nerve fibers. Am. J. Phys. 127, 131–139 (1939)
Keetels, M., Vroomen, J.: Perception of synchrony between the senses. Neural Bases Multisensory Process Murray MM, 1–61 (2012). https://doi.org/10.1201/b11092-12
Bushara, K.O., Grafman, J., Hallett, M.: Neural correlates of auditory – visual stimulus onset asynchrony detection. J. Neurosci. 21, 300–304 (2001)
Hirsh, I.J., Sherrick, C.E.: Perceived order in different sense modalities. J. Exp. Psychol. 62, 423–432 (1961)
Keetels, M., Vroomen, J.: Temporal recalibration to tactile-visual asynchronous stimuli. Neurosci. Lett. 430, 130–134 (2008). https://doi.org/10.1016/j.neulet.2007.10.044
Zampini, M., Shore, D.I., Spence, C.: Audiovisual prior entry. Neurosci. Lett. 381, 217–222 (2005). https://doi.org/10.1016/j.neulet.2005.01.085
Vatakis, A., Spence, C.: Audiovisual synchrony perception for music, speech, and object actions. 1 (2006). https://doi.org/10.1016/j.brainres.2006.05.078
Vatakis, A., Spence, C.: Audiovisual synchrony perception for speech and music assessed using a temporal order judgment task. 393, 40–44 (2006). https://doi.org/10.1016/j.neulet.2005.09.032
Stekelenburg, J.J., Vroomen, J.: Neural correlates of multisensory integration of ecologically valid audiovisual events. J. Cogn. Neurosci. 19, 1964–1973 (2007)
Conrey, B., Pisoni, D.B., Conrey, B., Pisoni, D.B.: Auditory-visual speech perception and synchrony detection for speech and nonspeech signals. J. Acoust. Soc. Am. 119, 4065–4073 (2006). https://doi.org/10.1121/1.2195091
Reed, T.E., Jensen, A.R.: Conduction velocity in a brain nerve pathway of normal adults correlates with intelligence level. Intelligence. 16, 259–272 (1992)
Moller, A.R., Colletti, V., Fiorino, F.G.: Neural conduction velocity of the human auditory nerve: bipolar recordings from the exposed intracranial portion of the eighth nerve during vestibular nerve section. Electroencephalogr. Clin. Neurophysiol. 92, 316–320 (1994)
Macefield, G., Gandevia, S.C., Burke, D.: Conduction velocities of muscle and cutaneous afferents in the upper and lower limbs of human subjects. Brain. 112, 1519–1532 (1989). https://doi.org/10.1093/brain/112.6.1519
Harrar, V., Harris, L.R.: Simultaneity constancy: detecting events with touch and vision. Exp. Brain Res. 166, 465–473 (2005). https://doi.org/10.1007/s00221-005-2386-7
Eisen, A.: Subject review: the somatosensory evoked potential. Can. J. Neurol. Sci. 9, 65 (1982)
Nakanishi, T., Takita, K., Toyokura, Y.: Somatosensory evoked responses to tactile tap in man. Electroencephalogr. Clin. Neurophysiol. 34, 1–6 (1973). https://doi.org/10.1016/0013-4694(73)90144-2
Gilley, P.M., Sharma, A., Dorman, M., Martin, K.: Developmental changes in refractoriness of the cortical auditory evoked potential. Clin. Neurophysiol. 116, 648–657 (2005). https://doi.org/10.1016/j.clinph.2004.09.009
Woods, D.L., Wyma, J.M., Yund, E.W., et al.: Factors influencing the latency of simple reaction time. 9, 1–12 (2015). https://doi.org/10.3389/fnhum.2015.00131
Jaśkowski, P., Jaroszyk, F., Hojan-Jezierska, D.: Temporal-order judgments and reaction time for stimuli of different modalities. Psychol. Res. 52, 35–38 (1990). https://doi.org/10.1007/BF00867209
Forster, B., Cavina-Pratesi, C., Aglioti, S.M.: Redundant target effect and intersensory facilitation from visual-tactile interactions in simple reaction time. Exp. Brain Res. 143, 480–487 (2002). https://doi.org/10.1007/s00221-002-1017-9
Goodall, E.V., Kosterman, L.M., Holsheimer, J., Struijk, J.J.: Modeling study of activation and propagation delays during stimulation of peripheral nerve fibers with a tripolar cuff electrode. IEEE Trans. Rehabil. Eng. 3, 272–282 (1995)
Wesselink, W.A., Holsheimer, J., Boom, H.B.K.: A model of the electrical behaviour of myelinated sensory nerve fibres based on human data. Med. Biol. Eng. Comput. 37, 228–235 (1999). https://doi.org/10.1007/BF02513291
Callier, T., Brantly, N., Caravalli, A., Bensmaia, S.J.: The frequency of cortical microstimulation shapes artificial touch. bioarxiv. (2019)
Oddo, C.M., Raspopovic, S., Artoni, F., et al.: Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. elife. 5, 1–27 (2016). https://doi.org/10.7554/eLife.09148
Christie, B., Graczyk, E., Charkhkar, H., et al.: Visuo-tactile synchrony of stimulation-induced sensation and natural somatosensation. J. Neural Eng. 16 (2019)
Ziegler-graham, K., Mackenzie, E.J., Ephraim, P.L., et al.: Estimating the prevalence of limb loss in the United States: 2005 to 2050. 89, 422–429 (2008). https://doi.org/10.1016/j.apmr.2007.11.005
Rybarczyk, B., Behel, J.: Limb loss and body image. In: Psychoprosthetics, pp. 23–31. Springer (2008)
Desmond, D.M.: Co**, affective distress, and psychosocial adjustment among people with traumatic upper limb amputations. J. Psychosom. Res. 62, 15–21 (2007). https://doi.org/10.1016/j.jpsychores.2006.07.027
Gallagher, P., Desmond, D., MacLachlan, M.: Psychoprosthetics. (2008)
Frank, R.G., Elliott, T.R.: Handbook of Rehabilitation Psychology. American Psychological Association (2000)
Briggs, W.: The mental health problems and needs of older people following lower-limb amputation. Rev. Clin. Gerontol. 16, 155 (2007). https://doi.org/10.1017/S0959259807002171
Williamson, G.M., Schulz, R., Bridges, M.W., Behan, A.M.: Social and psychological factors in adjustment to limb amputation. J. Soc. Behav. Pers. 9, 249 (1994)
Rybarczyk, B., Nyenhuis, D., Nicholas, J., et al.: Social discomfort and depression in a sample of adults with leg amputations. Arch. Phys. Med. Rehabil. 73, 1169–1173 (1992)
Eaton, W.W.: Epidemiologic evidence on the comorbidity of depression and diabetes. J. Psychosom. Res. 53, 903–906 (2002). https://doi.org/10.1016/S0022-3999(02)00302-1
Blazer, D.G., Kessler, R.C., McGonagle, K.A., Swartz, M.S.: The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am. J. Psychiatry. 151, 979–986 (1994)
Biddiss, E., Chau, T.: Upper-limb prosthetics: critical factors in device abandonment. Am. J. Phys. Med. Rehabil. 86, 977–987 (2007). https://doi.org/10.1097/PHM.0b013e3181587f6c
Biddiss, E., Chau, T.: Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthetics Orthot. Int. 31, 236–257 (2007)
Atkins, D., Heard, D.C.Y., Donovan, W.H.: Epidemiologic overview of individuals with upper-limb loss and their reported research priorities. J. Prosthetics Orthot. 8, 2–11 (1996)
Peerdeman, B., Boere, D., Witteveen, H., et al.: Myoelectric forearm prostheses: state of the art from a user-centered perspective. J. Rehabil. Res. Dev. 48, 719 (2011). https://doi.org/10.1682/JRRD.2010.08.0161
Burger, H., Vidmar, G.: A survey of overuse problems in patients with acquired or congenital upper limb deficiency. Prosthetics Orthot. Int. 40, 497–502 (2016). https://doi.org/10.1177/0309364615584658
Postema, S.G., Bongers, R.M., Brouwers, M.A., et al.: Musculoskeletal complaints in transverse upper limb reduction deficiency and amputation in the Netherlands: prevalence, predictors, and effect on health. Arch. Phys. Med. Rehabil. 97, 1137–1145 (2016). https://doi.org/10.1016/j.apmr.2016.01.031
Johansson, R., Westling, G.: Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp. Brain Res. 56, 550–564 (1984)
Johansson, R.S., Edin, B.B.: Predictive feed-forward sensory control during gras** and manipulation in man. Biomed. Res. 14, 95–106 (1993)
Häger-Ross, C., Johansson, R.S.: Nondigital afferent input in reactive control of fingertip forces during precision grip. Exp. Brain Res. 110, 131–141 (1996). https://doi.org/10.1007/BF00241382
Whitaker, T.A., Simões-Franklin, C., Newell, F.N.: Vision and touch: independent or integrated systems for the perception of texture? Brain Res. 1242, 59–72 (2008). https://doi.org/10.1016/j.brainres.2008.05.037
Srinivasan, M.A., LaMotte, R.H.: Tactual discrimination of softness. J. Neurophysiol. 73, 88–101 (1995). https://doi.org/10.1007/978-3-0348-9016-8_11
Childress, D.S.: Closed-loop control in prosthetic systems: historical perspective. Ann. Biomed. Eng. 8, 293–303 (1980)
Lewis, S., Russold, M.F., Dietl, H., Kaniusas, E.: User demands for sensory feedback in upper extremity prostheses. IEEE Int. Symp. Med. Meas. Appl. Proc. 2012, 1–4 (2012). https://doi.org/10.1109/MeMeA.2012.6226669
Lundberg, M., Hagberg, K., Bullington, J.: My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthetics Orthot. Int. 35, 207–214 (2011). https://doi.org/10.1177/0309364611409795
Hagberg, K., Häggström, E., Jönsson, S., et al.: Osseoperception and osseointegrated prosthetic limbs. Psychoprosthetics, 131–140 (2008)
Vargas, L., Whitehouse, G., Huang, H., et al.: Evoked haptic sensation in the hand with concurrent non-invasive nerve stimulation. IEEE Trans. Biomed. Eng. 66, 2761–2767 (2019). https://doi.org/10.1109/TBME.2019.2895575
D’Anna, E., Petrini, F.M., Artoni, F., et al.: A somatotopic bidirectional hand prosthesis with transcutaneous electrical nerve stimulation based sensory feedback. Sci. Rep. 7, 1–15 (2017). https://doi.org/10.1038/s41598-017-11306-w
Osborn, L.E., Dragomir, A., Betthauser, J.L., et al.: Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain. Sci Robot. 3, eaat3818 (2018). https://doi.org/10.1126/scirobotics.aat3818
Chandrasekaran, S., Nanivadekar, A.C., McKernan, G.P., et al.: Sensory restoration by epidural stimulation of dorsal spinal cord in upper-limb amputees. elife. 9, 1–26 (2020)
Schiefer, M.A., Tan, D.W., Sidik, S.M., et al.: Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J. Neural Eng. 13, 016001 (2016). https://doi.org/10.1088/1741-2560/13/1/016001
Horch, K., Meek, S., Taylor, T.G., Hutchinson, D.T.: Object discrimination with an artificial hand using electrical stimulation of peripheral tactile and proprioceptive pathways with intrafascicular electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 483–489 (2011). https://doi.org/10.1109/TNSRE.2011.2162635
Segil, J.L., Cuberovic, I., Graczyk, E.L., et al.: Combination of simultaneous artificial sensory percepts to identify prosthetic hand postures: a case study. Sci. Rep. 10, 1–15 (2020). https://doi.org/10.1038/s41598-020-62970-4
Mulvey, M.R., Fawkner, H.J., Radford, H.E., Johnson, M.I.: Perceptual embodiment of prosthetic limbs by transcutaneous electrical nerve stimulation. Neuromodulation. 15, 42–46; discussion 47 (2012). https://doi.org/10.1111/j.1525-1403.2011.00408.x
Mulvey, M.R., Radford, H.E., Fawkner, H.J., et al.: Transcutaneous electrical nerve stimulation for phantom pain and stump pain in adult amputees. Pain Pract. 13, 289–296 (2013). https://doi.org/10.1111/j.1533-2500.2012.00593.x
Graczyk, E.L., Gill, A., Tyler, D.J., Resnik, L.J.: The benefits of sensation on the experience of a hand: a qualitative case series. PLoS One. 14, 1–29 (2019). https://doi.org/10.1371/journal.pone.0211469
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Graczyk, E.L., Tyler, D.J. (2023). Somatosensory Neuromodulation with a Focus Towards Clinical Systems. In: Thakor, N.V. (eds) Handbook of Neuroengineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-5540-1_92
Download citation
DOI: https://doi.org/10.1007/978-981-16-5540-1_92
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5539-5
Online ISBN: 978-981-16-5540-1
eBook Packages: EngineeringReference Module Computer Science and Engineering