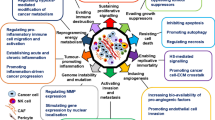
Abstract
Consolidated data indicate that tumor bulk is not only made up of a heterogeneous set of neoplastic cells but also of a variety of resident and infiltrating host cells, soluble factors, and components of the extracellular matrix which as a whole is defined as the tumor microenvironment. In this context, the extracellular matrix plays a fundamental role in tumor progression as it acts as a repository for various biomolecules such as growth factors, cytokines, enzymes, and inhibitors which are mainly linked to heparan sulfate proteoglycans (HSPG) and whose release can regulate the response or not of cancer cells. Among the various enzymes involved in the degradation of the ECM, heparanase (HPSE) has been shown to be particularly involved in tumor progression and metastatic invasion. This enzyme, capable of cutting heparan sulfate (HS) chains, is overexpressed in practically all solid tumors, clearly demonstrating that it has pro-invasive and pro-angiogenic characteristics for neoplastic cells.
Furthermore, considering that heparanase is released not only by tumor cells but also by platelets, endothelial cells, and immune cells, we can admit that its enzymatic activity has a strong impact on the tumor microenvironment. Here, we discuss the recent development in the study of heparanase in cancer progression as well as on novel mechanisms by which heparanase regulates the nature of the tumor microenvironment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, Joyce JA, Vlodavsky I (2008) Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem 283(26):18167–18176
Admyre C, Johansson SM, Qazi KR, Filén J-J, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179:1969–1978
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L (2002) Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360:295–305
Arasu UT, Kärnä R, Härkönen K, Oikari S, Koistinen A, Kröger H, Qu C, Lammi MJ, Rilla K (2017) Human mesenchymal stem cells secrete hyaluronan-coated extracellularvesicles. Matrix Biol 64:54–68. https://doi.org/10.1016/j.matbio.2017.05.001. Epub 2017 May 5
Au Yeung CL et al (2016) Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 7:11150
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P (2020) Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 18(1):59
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E et al (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14:677–685
Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, Mobley JA, Zhang Y, Brown EE, Vlodavsky I, Sanderson RD (2018) Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol 65:104–118. https://doi.org/10.1016/j.matbio.2017.09.001
Barash U, Zohar Y, Wildbaum G, Beider K, Nagler A, Karin N, Ilan N, Vlodavsky I (2014) Heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia 28(11):2178–2187
Bartolini B, Caravà E, Caon I, Parnigoni A, Moretto P, Passi A, Vigetti D, Viola M, Karousou E (2020) Heparan sulfate in the tumor microenvironment. Adv Exp Med Biol 1245:147–161
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D (2016) Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30:836–848. PubMed: 27960084
Beckhove P, Helmke BM, Ziouta Y, Bucur M, Dorner W, Mogler C et al (2005) Heparanase expression at the invasion front of human head and neck cancers and correlation with poor prognosis. Clin Cancer Res 11:2899–2906
Ben-Zaken O, Shafat I, Gingis-Velitski S, Bangio H, Kelson IK, Alergand T, Amor Y, Maya RB, Vlodavsky I, Ilan N (2008) Low and high affinity receptors mediate cellular uptake of heparanase. Int J Biochem Cell Biol 40(3):530–542
Boyango I, Barash U, Naroditsky I, Li JP, Hammond E, Ilan N, Vlodavsky I (2014) Heparanase cooperates with Ras to drive breast and skin tumorigenesis. Cancer Res 74(16):4504–4514
Brody I, Ronquist G, Gottfries A (1983) Ultrastructural localization of the prostasome—an organelle in human seminal plasma. Ups J Med Sci 88:63–80
Caruana I, Savoldo B, Hoyos V et al (2015) Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 21:524–529. https://doi.org/10.1038/nm.3833
Casu B, Naggi A, Torri G (2010) Heparin-derived heparan sulfate mimics to modulate heparan sulfate-protein interaction in inflammation and cancer. Matrix Biol 29(6):442–452
Cheng CC, Lee YH, Lin SP, Huangfu WC, Liu IH (2014) Cell-autonomous heparanase modulates self-renewal and migration in bone marrow-derived mesenchymal stem cells. J Biomed Sci 21:21
Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M (2013) Cancer cell exosomes depend on cell-surface heparin sulfate proteoglycans for their internalization and functional activity. PNAS 110(43):17380–17385. ww.pnas.org/cgi/doi/10.1073/pnas.1304266110
Collins LE, Troeberg L (2019) Heparan sulfate as a regulator of inflammation and immunity. J Leukoc Biol 105(1):81–92
Conigliaro A, Cicchini C (2019) Exosome-mediated signaling in epithelial to mesenchymal transition and tumor progression. J Clin Med 8:26. https://doi.org/10.3390/jcm8010026
Coombe DR, Gandhi NS (2019) Heparanase: a challenging cancer drug target. Front Oncol 9:article 1316. www.frontiersin.org. https://doi.org/10.3389/fonc.2019.01316
Cox TR (2021) The matrix in cancer. Nat Rev Cancer 21(4):217–238. https://doi.org/10.1038/s41568-020-00329-7
Dehne N, Mora J, Namgaladze D, Weigert A, Brüne B (2017) Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol 35:12–19. https://doi.org/10.1016/j.coph.2017.04.007
De Pasquale V, Pavone LM (2020) Heparan sulfate proteoglycan signaling in tumor microenvironment. Int J Mol Sci 21(18):6588
Di Gregorio J, Robuffo I, Spalletta S, Giambuzzi G, De Iuliis V, Toniato E, Martinotti S, Conti P, Flati V (2020) The epithelial-to-mesenchymal transition as a possible therapeutic target in fibrotic disorders. Front Cell Dev Biol 8:607483
Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A (1999) Matrix degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis 17:131–140
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implication for cancer. Nat Rev Mol Cell Biol 20(2):69–84
Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I (2004) Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis. J Natl Cancer Inst 96(16):1219–1230
Elkin M (2020) Role of heparanase in macrophage activation. Adv Exp Med Biol 1221:445–460
Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, Vlodavsky I (2001) Heparanase as mediator of angiogenesis: mode of action. FASEB J 15(9):1661–1663
Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, Hayward SW, Li D, Webb J (2017) Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. Cell Biol 216(11):3799–3816. https://doi.org/10.1083/jcb.201704053. Epub 2017 Oct 11
Fattet L et al (2020) Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev Cell 54:302–316.e7
Fears CY, Woods A (2006) The role of syndecans in disease and wound healing. Matrix Biol 25(7):443–456
Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I (1988) A heparin-binding angiogenic protein—basic fibroblast growth factor—is stored within basement membrane. Am J Pathol 130:393–400
Friand V, David G, Zimmermann P (2015) Syntenin and syndecan in the biogenesis of exosomes. Biol Cell 107:331–341. [PubMed: 26032692]
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14(10):1014–1022 5
Gialeli C, Theocharis AD, Karamanos NK (2011) Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278(1):16–27
Giese MA, Hind LE, Huttenlocher A (2019) Neutrophil plasticity in the tumor microenvironment. Blood 133(20):2159–2167
Goldberg R, Rubinstein AM, Gil N, Hermano E, Li JP, van der Vlag J, Atzmon R, Meirovitz A, Elkin M (2014) Role of heparanase-driven inflammatory cascade in pathogenesis of diabetic nephropathy. Diabetes 63(12):4302–4313
Goodall KJ, Poon IK, Phipps S, Hulett MD (2014) Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One 9(10):e109596
Hammond E, Khurana A, Shridhar V, Dredge K (2014) The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front Oncol 4:195
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322
Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SE, Wood MJ, Meisner-Kober NC (2016) Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol 213:173–184
Higashi N, Irimura T, Nakajima M (2020) Heparanase is involved in leukocyte migration. Adv Exp Med Biol 1221:435–444
Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Cancer Res 79(18):4557–4566
Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM (2013) Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5:1159–1168. [PubMed: 24290760]
Ilan N, Elkin M, Vlodavsky I (2006) Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol 38(12):2018–2039
Iozzo RV (2005) Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 6(8):646–656
Iozzo RV, Sanderson RD (2011) Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med 15:1013–1031
Iozzo RV, Schaefer L (2015) Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42:11–55
James R (2016) Edgar Q&A: what are exosomes, exactly? BMC Biol 14:46
Jayatilleke KM, Hulett MD (2020) Heparanase and the hallmarks of cancer. J Transl Med 18(1):453
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420
Jung O, Trapp-Stamborski V, Purushothaman A, ** H, Wang H, Sanderson RD, Rapraeger AC (2016) Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogenesis 5:e202. https://doi.org/10.1038/oncsis.2016.5
Kalluri R (2009) EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119:1417–1419
Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brézillon S, Götte M, Passi A, Vigetti D, Ricard-Blum S, Sanderson RD, Neill T, Iozzo RV (2018) Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem Rev 118(18):9152–9232
Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V, Ricard-Blum S, Schmelzer CEH, Duca L, Durbeej M, Afratis NA, Troeberg L, Franchi M, Masola V, Onisto M (2021) A guide to the composition and functions of the extracellular matrix. FEBS J. https://doi.org/10.1111/febs.15776. Epub ahead of print. PMID: 33605520
Khamaysi I, Singh P, Nasser S, Awad H, Chowers Y, Sabo E, Hammond E, Gralnek I, Minkov I, Noseda A, Ilan N, Vlodavsky I, Abassi Z (2017) The role of heparanase in the pathogenesis of acute pancreatitis: a potential therapeutic target. Sci Rep 7(1):715
Kim S-H, Turnbull J, Guimond S (2011) Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209:139–151
Kordelas L, Rebmann V, Ludwig A-K, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B (2014) MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28:970–973
Labani-Motlagh A, Ashja-Mahdavi M, Loskog A (2020) The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol 11:940
Larrue C, Saland E, Boutzen H, Vergez F, David M, Joffre C, Hospital MA, Tamburini J, Delabesse E, Manenti S, Sarry JE, Recher C (2016) Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells. Blood 127:882–892. [PubMed: 26286850]
Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, Doviner V, Rubinstein AM, Ishai-Michaeli R, Atzmon R, Sherman Y, Meirovitz A, Peretz T, Vlodavsky I, Elkin M (2011) Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest 121(5):1709–1721
Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17(9):528–542
Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N (2003) Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun 308(4):885–891
Li J, Pan Q, Rowan PD, Trotter TN, Peker D, Regal KM, Javed A, Suva LJ, Yang Y (2016) Heparanase promotes myeloma progression by inducing mesenchymal features and motility of myeloma cells. Oncotarget 7(10):11299–11309
Lim HC, Multhaupt HA, Couchman JR (2015) Cell surface proteoglycans control adhesion and invasion of breast carcinoma cells. Mol Cancer 14:15
Madhusoodanan J (2019) Matrix mimics cell shape studies. Nature 566:563–565
Manon-Jensen T, Itoh Y, Couchman JR (2010) Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J 277(19):3876–3889
Marzagalli M, Ebelt ND, Manuel ER (2019) Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol 59:236–250. https://doi.org/10.1016/j.semcancer.2019.08.002
Masola V, Maran C, Tassone E, Zin A, Rosolen A, Onisto M (2009) Heparanase activity in alveolar and embryonal rhabdomyosarcoma: implications for tumor invasion. BMC Cancer 9:304
Masola V, Gambaro G, Tibaldi E, Brunati AM, Gastaldello A, D’Angelo A, Onisto M, Lupo A (2012) Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem 287(2):1478–1488
Masola V, Zaza G, Secchi MF, Gambaro G, Lupo A, Onisto M (2014) Heparanase is a key player in renal fibrosis by regulating TGF-β expression and activity. Biochim Biophys Acta 1843(9):2122–2128
Masola V, Zaza G, Gambaro G, Onisto M, Bellin G, Vischini G, Khamaysi I, Hassan A, Hamoud S, Nativ O, Heyman SN, Lupo A, Vlodavsky I, Abassi Z (2016) Heparanase: a potential new factor involved in the renal epithelial mesenchymal transition (EMT) induced by ischemia/reperfusion (I/R) injury. PLoS One. 11(7):e016074
Masola V, Zaza G, Gambaro G, Franchi M, Onisto M (2020) Role of heparanase in tumor progression: molecular aspects and therapeutic options. Semin Cancer Biol 62:86–98
Massena S, Christoffersson G, Hjertström E, Zcharia E, Vlodavsky I, Ausmees N, Rolny C, Li JP, Phillipson M (2010) A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood 116(11):1924–1931
Mayes K, Elsayed Z, Alhazmi A, Waters M, Alkhatib SG, Roberts M, Song C, Peterson K, Chan V, Ailaney N, Malapati P, Blevins T, Lisnić B, Dumur CI, Landry JW (2017) BPTF inhibits NK cell activity and the abundance of natural cytotoxicity receptor co-ligands. Oncotarget 8(38):64344–64357
McKenzie E et al (2000) Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun 276(3):1170–1177
McMahon BJ, Kwaan HC (2015) Components of the plasminogen-plasmin system as biologic markers for cancer. Adv Exp Med Biol 867:145–156
Menzel K, Hausmann M, Obermeier F, Schreiter K, Dunger N, Bataille F et al (2006) Cathepsins B, L and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin Exp Immunol 146(1):169–180
Mustonen AM, Nieminen P, Joukainen A, Jaroma A, Kääriäinen T, Kröger H, Lázaro-Ibáñez E, Siljander PR, Kärjä V, Härkönen K, Koistinen A, Rilla K (2016) First in vivo detection and characterization of hyaluronan-coated extracellular vesicles in human synovial fluid. J Orthop Res 34:1960–1968
Mustonen AM, Capra J, Rilla K, Lehenkari P, Oikari S, Kääriäinen T, Joukainen A, Kröger H, Paakkonen T, Matilainen J, Nieminen P (2021) Characterization of hyaluronan-coated extracellular vesicles in synovial fluid of patients with osteoarthritis and rheumatoid arthritis. BMC Musculoskelet Disord 22:Article number 247
Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140(6):871–882
Noseda A, Barbieri P (2020) Roneparstat: development, preclinical and clinical studies. Adv Exp Med Biol 1221:523–538
Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R (2008) Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 31:1059–1062
Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, ** Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MC, Fuxe J (2016) TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 35(6):748–760
Peinado H, Lavotshkin S, Lyden D (2011) The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol 21:139–146. [PubMed: 21251983]
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y et al (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17:302–317
Piperigkou Z, Mohr B, Karamanos N, Götte M (2016) Shed proteoglycans in tumor stroma. Cell Tissue Res 365(3):643–655
Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101:13368–13373
Poon IK, Goodall KJ, Phipps S, Chow JD, Pagler EB, Andrews DM, Conlan CL, Ryan GF, White JA, Wong MK, Horan C, Matthaei KI, Smyth MJ, Hulett MD (2014) Mice deficient in heparanase exhibit impaired dendritic cell migration and reduced airway inflammation. Eur J Immunol 44(4):1016–1030
Properzi F, Logozzi M, Fais S (2013) Exosomes: the future of biomarkers in medicine. Biomark Med 7:769–778. https://doi.org/10.2217/bmm.13.63
Purushothaman A, Sanderson RD (2020) Heparanase: a dynamic promoter of myeloma progression. Adv Exp Med Biol 1221:331–349
Putz EM, Mayfosh AJ, Kos K, Barkauskas DS, Nakamura K, Town L, Goodall KJ, Yee DY, Poon IK, Baschuk N, Souza-Fonseca-Guimaraes F, Hulett MD, Smyth MJ (2017) NK cell heparanase controls tumor invasion and immune surveillance. J Clin Invest 127(7):2777–2788
Quail D, Joyce J (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437
Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, Sanderson RD (2013) The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J 280(10):2294–2306
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Reiland J, Sanderson RD, Waguespack M, Barker SA, Long R, Carson DD, Marchetti D (2004) Heparanase degrades syndecan-1 and perlecan heparan sulfate: functional implications for tumor cell invasion. J Biol Chem 279(9):8047–8055
Rilla K, Pasonen-Seppanen S, Deen AJ, Koistinen VV, Wojciechowski S, Oikari S et al (2013) HA production enhances shedding of plasma membrane-derived microvesicles. Exp Cell Res 319:2006–2018
Rilla K, Siiskonen H, Tammi M, Tammi R (2014) Hyaluronan-coated extracellular vesicles—a novel link between hyaluronan and cancer. Adv Cancer Res 123:121–148. https://doi.org/10.1016/B978-0-12-800092-2.00005-8
Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B et al (2011) SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res 17:1382–1393
Rodrigues-Junior DM, Pelarin MFA, Nader HB, Vettore AL, Pinhal MAS (2021) MicroRNA-1252-5p associated with extracellular vesicles enhances bortezomib sensitivity in multiple myeloma cells by targeting heparanase. OncoTargets Ther 14
Roucourt B, Meeussen S, Bao J, Zimmermann P, David G (2015) Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res 25:412–428
Ruan J, Trotter TN, Nan L, Luo R, Javed A, Sanderson RD, Suva LJ, Yang Y (2013) Heparanase inhibits osteoblastogenesis and shifts bone marrow progenitor cell fate in myeloma bone disease. Bone 57(1):10–17
Salem KZ, Moschetta M, Sacco A, Imberti L, Rossi G, Ghobrial IM, Manier S, Roccaro AM (2016) Exosomes in tumor angiogenesis. Methods Mol Biol 1464:25–34
Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I (2017) Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J 284(1):42–55. https://doi.org/10.1111/febs.13932
Sanderson RD, Bandari SK, Vlodavsky I (2019) Proteases and glycosidases on the surface of exosomes: newly discovered mechanisms for extracellular remodeling. Matrix Biol 75–76:160–169
Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18:1217–1223
Shteingauz A, Boyango I, Naroditsky I, Hammond E, Gruber M, Doweck I, Ilan N, Vlodavsky I (2015) Heparanase enhances tumor growth and chemoresistance by promoting autophagy. Cancer Res 75:3946–3957. [PubMed: 26249176]
Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG (2012) Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol 181(6):1895–1899
Simizu S, Ishida K, Wierzba MK, Osada H (2004) Secretion of heparanase protein is regulated by glycosylation in human tumor cell lines. J Biol Chem 279(4):2697–2703
Singel KL, Segal BH (2016) Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev 273(1):329–343
Spiegel A, Zcharia E, Vagima Y, Itkin T, Kalinkovich A, Dar A, Kollet O, Netzer N, Golan K, Shafat I, Ilan N, Nagler A, Vlodavsky I, Lapidot T (2008) Heparanase regulates retention and proliferation of primitive Sca-1+/c-kit+/Lin- cells via modulation of the bone marrow microenvironment. Blood 111(10):4934–4943
Spyrou A, Kundu S, Haseeb L, Yu D, Olofsson T, Dredge K, Hammond E, Barash U, Vlodavsky I, Forsberg-Nilsson K (2017) Inhibition of heparanase in pediatric brain tumor cells attenuates their proliferation, invasive capacity, and in vivo tumor growth. Mol Cancer Ther 16:1705–1716
Stetler-Stevenson WG, Yu AE (2001) Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol 11(2):143–152
Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA (2006) Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer 42(6):768–778
Szebeni GJ, Vizler C, Kitajka K, Puskas LG (2017) Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm 2017:9294018
Tang W, Nakamura Y, Tsujimoto M, Sato M, Wang X, Kurozumi K et al (2002) Heparanase: a key enzyme in invasion and metastasis of gastric carcinoma. Mod Pathol 15:593–598
Theodoro TR, Matos LL, Cavalheiro RP, Justo GZ, Nader HB, Pinhal MAS (2019) Crosstalk between tumor cells and lymphocytes modulates heparanase expression. J Transl Med 17:103. https://doi.org/10.1186/s12967-019-1853-z
Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD (2013) Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 288:10093–10099
Turturici G, Tinnirello R, Sconzo G, Geraci F (2014) Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol 306:C621–C633
Vella LJ, Greenwood DL, Cappai R, Scheerlinck JP, Hill AF (2008) Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet Immunol Immunopathol 124:385–393
Vlodavsky I, Miao HQ, Medalion B, Danagher P, Ron D (1996) Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev 15:177–186
Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I (1999) Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med 5(7):793–802
Vlodavsky I, Beckhove P, Lerner I, Pisano C, Meirovitz A, Ilan N, Elkin M (2012) Significance of heparanase in cancer and inflammation. Cancer Microenviron 5(2):115–132
Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N (2016) Heparanase: from basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat 29:54–75. https://doi.org/10.1016/j.drup.2016.10.001
Vlodavsky I, Ilan N, Sanderson RD (2020) Forty years of basic and translational heparanase research. In: Heparanase. Chapter first online: 10 April 2020 part of the Advances in experimental medicine and biology book series (AEMB, vol 1221). Springer, Cham, pp 3–59
Waterman M, Ben-Izhak O, Eliakim R, Groisman G, Vlodavsky I, Ilan N (2007) Heparanase upregulation by colonic epithelium in inflammatory bowel disease. Mod Pathol 20(1):8–14
Weissmann M, Arvatz G, Horowitz N, Feld S, Naroditsky I, Zhang Y et al (2016) Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis. Proc Natl Acad Sci U S A 113:704–709
Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM (2009) Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 136:320–330
Wong SY, Hynes RO (2006) Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 5(8):812–817. https://doi.org/10.4161/cc.5.8.2646. Epub 2006 Apr 17
Wu L, Viola CM, Brzozowski AM, Davies GJ (2015) Structural characterization of human heparanase reveals insights into substrate recognition. Nat Struct Mol Biol 22(12):1016–1022
**e M, Li J-P (2019) Heparan sulfate proteoglycan—a common receptor for diverse cytokines. Cell Signal 54:115–121
Yang N, Friedl A (2016) Syndecan-1-Induced ECM Fiber alignment requires integrin αvβ3 and Syndecan-1 ectodomain and heparan sulfate chains. PLoS One 11(2):e0150132
Zahavi T, Salmon-Divon M, Salgado R, Elkin M, Hermano E, Rubinstein AM, Francis PA, Di Leo A, Viale G, de Azambuja E, Ameye L, Sotiriou C, Salmon A, Kravchenko-Balasha N, Sonnenblick A (2021) Heparanase: a potential marker of worse prognosis in estrogen receptor-positive breast cancer. NPJ Breast Cancer 7(1):67. https://doi.org/10.1038/s41523-021-00277-x
Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, Weinstein T, Li JP, Lindahl U, Vlodavsky I (2004) Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J 18(2):252–263
Zhang HG, Grizzle WE (2014) Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol 184:28–41. [PubMed: 24269592]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Masola, V., Greco, N., Gambaro, G., Franchi, M., Onisto, M. (2022). Heparanase: A Paramount Enzyme for Cancer Initiation, Progression, and Metastasis. In: Kovalszky, I., Franchi, M., Alaniz, L.D. (eds) The Extracellular Matrix and the Tumor Microenvironment. Biology of Extracellular Matrix, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-99708-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-99708-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99707-6
Online ISBN: 978-3-030-99708-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)