Abstract
The objectives of this research are twofold: (a) to review current methods for rapid analysis of dissolved protein in water and wastewater and (b) to introduce a new spectrophotometrical method for determination of dissolved proteins in the 10–2500 mg/L range using carbonate-tartrate reagent, phosphomolybdic-phosphotungstic reagent, and copper sulfate.
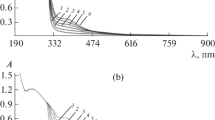
The new analytical method is a colorimetric determination based on the reaction of dissolved protein, specifically the peptide bonds and the amino acids tryptophan and tyrosine with the authors’ suggested reagents. Two reactions are necessary: (a) the reaction between the protein molecules and copper ions in an alkaline solution and (b) the reduction of a phosphomolybdic-phosphotungstic reagent by the copper-treated protein. The optical density of treated dissolved protein is then determined at 400–700 nm range using a spectrophotometer with 1 cm light path or longer. With certain modification, this method may also be used in the field using the calibration curves prepared in the laboratory. Flotation process is highly efficient for removal of dissolved proteins from wastewater. Therefore, determination of dissolved proteins in water is an important task to environmental scientists.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- Br:
-
Bromine
- CuSO4:
-
Copper sulfate
- HCl:
-
Hydrochloric acid
- H3PO4:
-
Phosphoric acid
- KNOX:
-
Knox Gelatin Inc.
- Li2SO4:
-
Lithium sulfate
- NaOH:
-
Sodium hydroxide, caustic soda
- Na2CO3:
-
Sodium carbonate
- Na2C4H4O6-2H2O:
-
Sodium tartrate
- Na2MoO4-2H2O:
-
Sodium molybdate
- Na2WO4-2H2O:
-
Sodium tungstate
References
Woods C (1965) Determination of proteins in waste water. Technical Paper presented at Cornell University, Ithica, NY.
Angell AR, Mata L, de Nys R, Paul NA (2016) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J Appl Phycol 28:511–524. https://doi.org/10.1007/s10811-015-0650-1
Imafidon GI, Sosulski FW (1990) Non-protein nitrogen contents of animal and plant foods. J Agric Food Chem 38:114–118. https://doi.org/10.1021/jf00091a023.6
Jones DB (1941) Factors for converting percentages of nitrogen in foods and feeds into percentages of protein. US Department of Agriculture, Washington, DC
Mariotti F, Tome D, Mirand PP (2008) Converting nitrogen into protein—beyond 6.25 and Jones’ factors. Crit Rev Food Sci 48:177–184. https://doi.org/10.1080/10408390701279749
Alhamdani MSS, Schröder C, Werner J, Giese N, Bauer A, Hoheisel JD (2010) Single-step procedure for the isolation of proteins at near-native conditions from mammalian tissue for proteomic analysis on antibody microarrays. J Proteome Res 9:963–971. https://doi.org/10.1021/pr900844q
Maehre HK, Edvinsen GK, Eilertsen KE, Elvevoll EO (2016) Heat treatment increases the protein bioaccessibility in the red seaweed dulse (Palmaria palmata), but not in the brown seaweed winged kelp (Alaria. esculenta). J Appl Phycol 28:581–590
Moore S, Stein WH (1963) Chromatographic determination of amino acids by the use of automatic recording equipment. Method Enzymol 6:819–831
Maehre HK, Hamre K, Elvevoll EO (2013) Nutrient evaluation of rotifers and zooplankton: feed for marine fish larvae. Aquac Nutr 19:301–311. https://doi.org/10.1111/j.1365-2095.2012.00960.x
Latimer GW (2016) Official methods of analysis of AOAC international. AOAC International, Gaithersburg
Kjeldahl J (1883) Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius J Anal Chem 22:366–382. https://doi.org/10.1007/BF01338151
Lourenço SO, Barbarino E, De-Paula JC, Pereira LOdS, Lanfer Marquez UM (2002) Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Res 50:233–241. https://doi.org/10.1111/j.1440-1835.2002.tb00156.x
Hartree EF (1972) Determination of protein—modification of Lowry method that gives a linear photometric response. Anal Biochem 48:422–427. https://doi.org/10.1016/0003-2697(72)90094-2
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Pickering MV, Newton P (1990) Amino acid hydrolysis—old problems, new solutions. LC GC Mag Sep Sci 8:778–780
FAO (2003) Food energy—methods of analysis and conversion factors. Food and Agriculture Organization of the United Nations, Rome
Dumas JBA (1831) Procedes de l’analyse organique. Ann Chim Phys T47:198–213
Sosulski FW, Imafidon GI (1990) Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J Agric Food Chem 38:1351–1356. https://doi.org/10.1021/jf00096a011
Moore JC, DeVries JW, Lipp M, Griffiths JC, Abernethy DR (2010) Total protein methods and their potential utility to reduce the risk of food protein adulteration. Compr Rev Food Sci F 9:330–357. https://doi.org/10.1111/j.1541-4337.2010.00114.x
Ingelfinger JR (2008) Melamine and the global implications of food contamination. N Engl J Med 359:2745–2748. https://doi.org/10.1056/NEJMp0808410
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28. https://doi.org/10.1016/S0924-2244(99)00015-1
Taboada C, Millan R, Miguez I (2013) Evaluation of marine algae Undaria pinnatifida and Porphyra purpurea as a food supplement: composition, nutritional value and effect of intake on intestinal, hepatic and renal enzyme activities in rats. J Sci Food Agric 93:1863–1868. https://doi.org/10.1002/jsfa.5981
Biancarosa I, Espe M, Bruckner CG, Heesch S, Liland N, Waagbo R, Torstensen B, Lock EJ (2017) Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J Appl Phycol 29:1001–1009. https://doi.org/10.1007/s10811-016-0984-3
Maehre HK, Malde MK, Eilertsen KE, Elvevoll EO (2014) Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J Sci Food Agric 94:3281–3290. https://doi.org/10.1002/jsfa.6681
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K (eds) Seaweed biology: novel insights into ecophysiology, ecology and utilization. Springer, Heidelberg, pp 87–107
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB (2010) Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J Agric Food Chem 58:8139–8144. https://doi.org/10.1021/jf1005935
Lleu PL, Rebel G (1991) Interference of Good buffers and other biological buffers with protein determination. Anal Biochem 192:215–218. https://doi.org/10.1016/0003-2697(91)90210-K
Mæhre HK, Guro L, Edvinsen K, Elvevoll EO, Jensen IJ (2018) Protein determination—method matters. Foods 7(1):5. https://doi.org/10.3390/foods7010005. www.ncbi.nlm.nih.gov/pmc/articles/PMC5789268/
Protein biology resource library, overview of protein assays methods (2019). www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-protein-assays.html
University of Massachusetts, Amhurst, MA, USA. Analysis of protein (2019). https://people.umass.edu/~mcclemen/581Proteins.html
Wang MHS, Wang LK, DeMichele E (2018) BOD determination, cleaning solution preparation and waste disposal in laboratories. In: Wang LK, MHS W, Hung YT, Shammas NK, Chen JP (eds) Handbook of dvanced industrial and hazardous wastes management. CRC Press, Taylor & Francis Group, Boca Raton, FL, USA, pp 797–808
Wang MHS, Wang LK, DeMichele E (2018) Principles, procedures and heavy metal management of dichromate reflux method for COD determination in laboratories. In: Wang LK, MHS W, Hung YT, Shammas NK, Chen JP (eds) Handbook of advanced industrial and hazardous wastes management. CRC Press, Boca Raton, FL, USA, pp 809–818
Goel RK, Flora JRV, Chen JP (2004) Flow equalization and neutralization. In: Wang LK, Hung YT, Shammas NK (eds) Physeicochemical treatment processes. Humana, Press, Totowa, NJ, USA, pp 21–46
Wang MHS, Wang LK (2015) Environmental water engineering glossary. In: Yang CT, Wang LK (eds) Advances in water resources engineering. Springer, New York, USA, pp 471–556
HACH Company (2002) Water analysis handbook, 4th edn. HACH Company, Loveland, USA, p 1260
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Glossary [30, 34, 35]
Glossary [30, 34, 35]
- Absolute method: :
-
A body of procedures and techniques for which measurement is based entirely on physically defined, fundamental quantities.
- Accuracy: :
-
The degree of agreement between an observed value and an accepted reference value. Accuracy includes a combination of random error (precision) and systematic error (bias) components, which are due to sampling and analytical operations; it is a data quality indicator. USEPA recommends that this term not be used and that precision and bias be used to convey the information usually associated with accuracy.
- Acid: :
-
Any substance capable of giving up a proton; a substance that ionizes in solution to give the positive ion of the solvent; a solution with a pH measurement less than 7.
- Acidity: :
-
The quantitative capacity of aqueous solutions to react with hydroxyl ions. It is measured by titration with a standard solution of base to a specified end point.
- Aliquot: :
-
A subsample derived by a divisor that divides a sample into a number of equal parts and leaves no remainder; a subsample resulting from such a division. In analytical chemistry, the term “aliquot” is generally used to define any representative portion of the sample.
- Alkalinity: :
-
The capacity of water to neutralize acids, a property imparted by the water’s content of carbonate, bicarbonate, hydroxide, and on occasion borate, silicate, and phosphate. It is expressed in milligrams per liter of equivalent calcium carbonate (mg/L CaCO3).
- Analysis (chemical): :
-
The determination of the qualitative and/or quantitative composition of a substance.
- Analyte: :
-
The substance, a property of which is to be measured by chemical analysis.
- Analytical batch: :
-
A group of samples, including quality control samples, which are processed together using the same method, the same lots of reagents, and at the same time or in continuous, sequential time periods. Samples in each batch should be of similar composition and share common internal quality control standards.
- Analytical reagent (AR): :
-
The American Chemical Society’s designation for the highest purity of certain chemical reagents and solvents.
- Anhydrous: :
-
A term meaning without water.
- Background level (environmental): :
-
The concentration of substance in a defined control area during a fixed period of time before, during, or after a data-gathering operation.
- Base: :
-
Any substance that contains hydroxyl (OH) groups and furnishes hydroxide ions in solution; a molecular or ionic substance capable of combining with a proton to form a new substance; a substance that provides a pair of electrons for a covalent bond with an acid; a solution with a pH of greater than 7.
- Batch: :
-
A quantity of material produced or processed in one operation, considered to be a uniform discrete unit.
- Blank sample: :
-
A clean sample or a sample of matrix processed so as to measure artifacts in the measurement (sampling and analysis) process.
- Calibrate: :
-
To determine, by measurement or comparison with a standard, the correct value of each scale reading on a meter or other device, or the correct value for each setting of a control knob. The levels of the calibration standards should bracket the range of planned measurements.
- Calibration curve: :
-
The graphical relationship between the known values for a series of calibration standards and instrument responses.
- Calibration standard: :
-
A substance or reference material used to calibrate an instrument.
- Calibration: :
-
The checking, adjusting, or systematic standardizing of the graduations of a quantitative measuring instrument.
- Candidate method: :
-
A body of procedures and techniques of sample collection and/or analysis that is submitted for approval as a reference method, an equivalent method, or an alternative method.
- Caustic soda: :
-
Sodium hydroxide, NaOH.
- Caustic: :
-
Capable of destroying or eating away by chemical action; a hydroxide of a light metal.
- Check sample: :
-
An uncontaminated sample matrix spiked with known amounts of analytes usually from the same source as the calibration standards. It is generally used to establish the stability of the analytical system but may also be used to assess the performance of all or a portion of the measurement system.
- Check standard: :
-
A substance or reference material obtained from a source independent from the source of the calibration standard; used to prepare check samples.
- Chemical analysis: :
-
The use of a standard chemical analytical procedures to determine the concentration of a specific analyte in a sample, or qualitatively or quantitatively measure a specific parameter of a sample.
- Clean sample: :
-
A sample of a natural or synthetic matrix containing no detectable amount of the analyte of interest and no interfering material.
- Colorimeters: :
-
A colorimeter is designed to perform a type of psychophysical sample analysis by mimicking human eye–brain perception or designed to see color the way we do. If desired, this data may be compared to a standard or reference to determine acceptability. Colorimeters are accurate for straightforward color measurement and ideally suited for determination of color difference, fastness, and strength as well as routine comparisons of similar colors. They can be invaluable for color quality control and are primarily used in the production and inspection phases of manufacturing. While colorimeters can produce highly accurate color measurements, they also have several shortcomings; they are not able to identify metamerism or colorant strength, are not ideally suited for color formulation, and cannot be used under variable illuminant/observer conditions.
- Composite sample: :
-
A sample prepared by physically combining two or more samples having some specific relationship and processed to ensure homogeneity.
- Concentration: :
-
In solutions, the mass, volume, or number of moles of solute present in proportion to the amount of solvent or total solution. Common measures are molarity, normality, percent, molality, and specific gravity scales.
- Contamination: :
-
A general term signifying the introduction into water of microorganisms, chemicals, wastes, or sewage, which renders the water unfit for its intended use.
- Data: :
-
Facts or figures from which conclusions can be inferred.
- Detection limit (DL): :
-
The lowest concentration or amount of the target analyte that can be determined to be different from zero by a single measurement at a stated level of probability.
- Determination: :
-
The application of the complete analytical process of measuring the property of interest in a sample, from selecting or measuring a test portion to the reporting of results.
- Diluent: :
-
A substance added to another to reduce the concentration and resulting in a homogeneous end product without chemically altering the compound of interest.
- Dilution factor: :
-
The numerical value obtained from dividing the new volume of a diluted substance by its original volume.
- Dissolved protein: :
-
Protein substances that can dissolve in water.
- Distilled water: :
-
Water that has been purified by distillation (boiling the water off as steam and condensing it back to a liquid, leaving the impurities behind). Having been boiled, it is also sterile.
- Document control: :
-
A systematic procedure for indexing documents by number, date, and revision number for archiving, storage, and retrieval.
- Duplicate analyses or measurements: :
-
The analyses or measurements of the variable of interest performed identically on two subsamples of the same sample. The results from duplicate analyses are used to evaluate analytical or measurement precision but not the precision of sampling, preservation, or storage internal to the laboratory.
- Duplicate samples: :
-
Two samples taken from and representative of the same population and carried through all steps of the sampling and analytical procedures in an identical manner. Duplicate samples are used to assess variance of the total method, including sampling and analysis.
- Duplicate: :
-
An adjective describing the taking of a second sample or performance of a second measurement or determination. Often incorrectly used as a noun and substituted for “duplicate sample.” Replicate is to be used if there are more than two items.
- Environmental sample: :
-
A sample of any material that is collected from an environmental source.
- Environmentally related measurement: :
-
Any assessment of environmental concern generated through or for field, laboratory, or modeling processes; the value obtained from such an assessment.
- Equivalent method: :
-
Any method of sampling and/or analysis demonstrated to result in data having a consistent and quantitatively known relationship to the results obtained with a reference method under specified conditions and formally recognized by the USEPA.
- Error (measurement): :
-
The difference between an observed or corrected value of a variable and a specified, theoretically correct, or true value.
- External quality control: :
-
The activities that are routinely initiated and performed by persons outside of normal operations to assess the capability and performance of a measurement process.
- Field blank: :
-
A clean sample (e.g., distilled water), carried to the sampling site, exposed to sampling conditions (e.g., bottle caps removed, preservatives added) and returned to the laboratory and treated as an environmental sample. Field blanks are used to check for analytical artifacts and/or background introduces by sampling and analytical procedures.
- Filtration: :
-
The process of separating solids from a liquid by means of a porous substance through which only the liquid can pass.
- Good laboratory practices (GLP): :
-
Either general guidelines or formal regulations for performing basic laboratory operations or activities that are known or believed to influence the quality and integrity of the results.
- Grab sample: :
-
A single sample that is collected at one point in time and place.
- Hazardous wastes: :
-
A hazardous waste is a material that is subject to special consideration by the USEPA, under 40CFR261. State or local authorities may also designate additional materials as hazardous waste in their areas. The definition given by 40 CFR 261 defines a hazardous waste as a solid waste that is not excluded from regulation and meets one or more of the following criteria: (a) it is a discarded commercial chemical product, off-specification species, container residue, or spill residue of materials specifically listed in 40CFR261.33 (P- and U-codes); (b) it is a waste from a specific source listed in 40CFR261.32 (K-code); (c) it is a waste from a nonspecific source listed in 40CFR261.31 (F-code); and/or (d) it displays any of the following characteristics of hazardous wastes: ignitability (such as flash point is below 60 degree C or 140 degree F; it is classified by DOT as an oxidizer D001), corrosivity (such as the pH of the waste material is less than or equal to 2, or greater than or equal to 12.5, or classified by DOT as D002), reactivity (such as the waste material is unstable, reacts violently with water, may generate toxic gases when mixed with water, or classified by DOT as D003), or toxicity (such as it is classified by DOT as D004-D043).
- Instrument blank: :
-
A clean sample processed through the instrumental steps of the measurement process; used to determine instrument contamination. See Dynamic blank.
- Interference: :
-
A positive or negative effect on a measurement caused by a variable other than the one being investigated.
- Kjeldahl method: :
-
The Kjeldahl method was developed in 1883 by a brewer called Johann Kjeldahl. A food is digested with a strong acid so that it releases nitrogen, which can be determined by a suitable titration technique. The amount of protein present is then calculated from the nitrogen concentration of the food. The Kjeldahl method does not measure the protein content directly; a conversion factor (F) is needed to convert the measured nitrogen concentration to a protein concentration. A conversion factor of 6.25 (equivalent to 0.16 g nitrogen per gram of protein) is used for many applications; however, this is only an average value, and each protein has a different conversion factor depending on its amino acid composition. The Kjeldahl method can conveniently be divided into three steps: digestion, neutralization, and titration.
- Management systems review (MSR): :
-
The qualitative assessment of a data collection operation and/or organization(s) to establish whether the prevailing quality management structure, practices, and procedures are adequate for ensuring that the type and quality of data needed and expected are obtained.
- Matrix: :
-
A specific type of medium (e.g., surface water, drinking water) in which the analyte of interest may be contained.
- Measurement range: :
-
The range over which the precision and/or recovery of a measurement method is regarded as acceptable.
- Measurement standard: :
-
A standard added to the prepared test portion of a sample (e.g., to the concentrated extract or the digestate) as a reference for calibrating and controlling measurement or instrumental precision and bias.
- Median: :
-
The middle value for an ordered set of n values; represented by the central value when n is odd or by the mean of the two most central values when n is even.
- Medium: :
-
A substance (e.g., air, water, soil) which serves as a carrier of the analytes of interest.
- Method: :
-
A body of procedures and techniques for performing a task (e.g., sampling, characterization, quantification) systematically presented in the order in which they are to be executed.
- Optical density: :
-
It is measured in a spectrophotometer; can be used as a measure of the concentration of substances in a suspension. As visible light passes through a cell suspension, the light is scattered. Greater scatter indicates that more bacteria or other material is present. Optical density measures the amount of attenuation, or intensity lost, when light passes through an optical component. It also tracks attenuation based on the scattering of light, whereas absorbance considers only the absorption of light within the optical component.
- Parameter: :
-
Any quantity such as a mean or a standard deviation characterizing a population. Commonly misused for “variable,” “characteristic,” or “property.”
- pH adjustment: :
-
A means of maintaining the optimum pH through the use of chemical additives.
- pH: :
-
The negative logarithm of the hydrogen ion concentration (−log10[H+]), where H+ is the hydrogen-ion concentration in moles per liter. Neutral water has a pH value of 7.
- Precision: :
-
The degree to which a set of observations or measurements of the same property, usually obtained under similar conditions, conform to themselves; a data quality indicator. Precision is usually expressed as standard deviation, variance, or range, in either absolute or relative terms.
- Procedure: :
-
A set of systematic instructions for performing an operation.
- Protein: :
-
Proteins are polymers of amino acids. Twenty different types of amino acids occur naturally in proteins. Proteins differ from each other according to the type, number, and sequence of amino acids that make up the polypeptide backbone. As a result, they have different molecular structures, nutritional attributes, and physiochemical properties. Proteins are important constituents of foods for a number of different reasons. They are a major source of energy, as well as contain essential amino acids, such as lysine, tryptophan, methionine, leucine, isoleucine, and valine, which are essential to human health, but which the body cannot synthesize. Proteins are also the major structural components of many natural foods, often determining their overall texture, for example, tenderness of meat or fish products. Isolated proteins are often used in foods as ingredients because of their unique functional properties, that is, their ability to provide desirable appearance, texture, or stability.
- Quality assurance (QA): :
-
An integrated system of activities involving planning, quality control, quality assessment, reporting, and quality improvement to ensure that a product or service meets defined standards of quality with a stated level of confidence.
- Quality control (QC): :
-
The overall system of technical activities whose purpose is to measure and control the quality of a product or service so that it meets the needs of users. The aim is to provide quality that is satisfactory, adequate, dependable, and economical.
- Quality: :
-
The sum of features and properties/characteristics of a product or service that bear on its ability to satisfy stated needs.
- Random: :
-
Lacking a definite plan, purpose, or pattern; due to chance.
- Range: :
-
The difference between the minimum and the maximum of a set of values.
- Raw data: :
-
Any original factual information from a measurement activity or study recorded in laboratory worksheets, records, memoranda, notes, or exact copies thereof and that are necessary for the reconstruction and evaluation of the report of the activity or study. Raw data may include photographs; microfilm or microfiche copies; computer printouts; magnetic media, including dictated observations; and recorded data from automated instruments. If exact copies of raw data have been prepared (e.g., tapes that have been transcribed verbatim, dated, and verified accurate by signature), the exact copy or exact transcript may be substituted.
- Reagent blank: :
-
A sample consisting of reagent(s), without the target analyte or sample matrix, introduced into the analytical procedure at the appropriate point and carried through all subsequent steps to determine the contribution of the reagents and of the involved analytical steps to error in the observed value.
- Reagent grade: :
-
The second highest purity designation for reagents that conform to the current specifications of the American Chemical Society Committee on Analytical Reagents.
- Reagent: :
-
A chemical substance used to cause a reaction for the purpose of chemical analysis.
- Reduction treatment: :
-
The opposite of oxidation treatment wherein a reductant is used to lower the valence state of a pollutant to a less-toxic form, for example, the use of SO2 to reduce Cr6+ to Cr3+ in an acidic solution.
- Reduction: :
-
Chemical reaction in which an atom or a molecule gains an electron; decrease in positive valence; addition of hydrogen to a molecule.
- Sample variance (statistical): :
-
A measure of the dispersion of a set of values. The sum of the squares of the difference between the individual values of a set and the arithmetic mean of the set, divided by one less than the number of values in the set. (The square of the sample standard deviation.)
- Sample: :
-
A part of a larger whole or a single item of a group; a finite part or subset of a statistical population. A sample serves to provide data or information concerning the properties of the whole group or population.
- Sampling: :
-
The process of obtaining a representative portion of the material of concern.
- Solution: :
-
A liquid (solvent) that contains a dissolved substance (solute).
- Solvent: :
-
A liquid used to dissolve another substance.
- Spectrophotometers: :
-
A spectrophotometer is an instrument designed for physical sample analysis via full-spectrum color measurement. By providing wavelength-by-wavelength spectral analysis of a sample’s reflectance, absorbance, or transmittance properties, it produces precise data beyond that observable by the human eye. Spectrophotometers offer a higher level of flexibility and versatility than colorimeters due in part to the fact that they offer multiple illuminant/observer combinations and are capable of measuring metamerism, identifying colorant strength, analyzing a comprehensive range of sample types, and giving users a choice between including or excluding specular reflectance to account for geometric attributes. Full-spectrum analysis also provides for greater specificity, potentially identifying color differences missed by colorimeters.
- Spiked reagent blank: :
-
A specified amount of reagent blank fortified with a known mass of the target analyte; usually used to determine the recovery efficiency of the method.
- Spiked sample: :
-
A sample prepared by adding a known mass of target analyte to a specified amount of matrix sample for which an independent estimate of target analyte concentration is available. Spiked samples are used, for example, to determine the effect of the matrix on a method’s recovery efficiency.
- Split samples: :
-
Two or more representative portions taken from a sample or subsample and analyzed by different analysts or laboratories. Split samples are used to replicate the measurement of the variable(s) of interest.
- Standard (measurement): :
-
A substance or material with a property quantified with sufficient accuracy to permit its use to evaluate the same property in a similar substance or material. Standards are generally prepared by placing a reference material in a matrix.
- Standard addition: :
-
The procedure of adding known increments of the analyte of interest to a sample to cause increases in detection response. The level of the analyte of interest present in the original sample is subsequently established by extrapolation of the plotted responses.
- Standard deviation: :
-
The most common measure of the dispersion or imprecision of observed values expressed as the positive square root of the variance.
- Standard method: :
-
An assemblage of techniques and procedures based on consensus or other criteria, often evaluated for its reliability by collaborative testing and receiving organizational approval.
- Standard operating procedure (SOP): :
-
A written document that details the method of an operation, analysis, or action whose techniques and procedures are thoroughly prescribed and that is accepted as the method for performing certain routine or repetitive tasks.
- Standard reference material (SRM): :
-
A certified reference material produced by the U.S. National Institute of Standards and Technology and characterized for absolute content independent of analytical method.
- Standard solution: :
-
A solution containing a known concentration of analytes, prepared and verified by a prescribed method or procedure and used routinely in an analytical method.
- UV-visible spectroscopy: :
-
A number of methods have been devised to measure protein concentration, which are based on UV-visible spectroscopy. Either these methods use the natural ability of proteins to absorb (or scatter) light in the UV-visible region of the electromagnetic spectrum, or they chemically or physically modify proteins to make them absorb (or scatter) light in this region. The basic principle behind each of these tests is similar. First of all, a calibration curve of absorbance (or turbidity) versus protein concentration is prepared using a series of protein solutions of known concentration. The absorbance (or turbidity) of the solution being analyzed is then measured at the same wavelength, and its protein concentration determined from the calibration curve. The main difference between the tests are the chemical groups that are responsible for the absorption or scattering of radiation, for example, peptide bonds, aromatic side groups, basic groups, and aggregated proteins.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wang, L.K., Wang, MH.S., Thayer, A.E. (2021). A Spectrophotometrical Method for Determination of Dissolved Proteins in Water or Wastewater. In: Wang, L.K., Wang, MH.S., Shammas, N.K., Aulenbach, D.B. (eds) Environmental Flotation Engineering. Handbook of Environmental Engineering, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-030-54642-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-54642-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54640-3
Online ISBN: 978-3-030-54642-7
eBook Packages: EngineeringEngineering (R0)