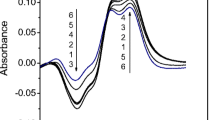
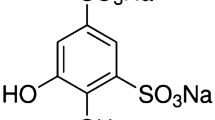
Abstract
In this study, we developed a simple method that enables iron(III) in environmental water to be directly determined via spectrophotometry. In water samples, iron(III) formed a yellowish complex with N-1-Naphthylethylenediamine dihydrochloride (NEDA) at pH 2.0–2.8, the maximum absorption wavelength of which was 462 nm. Detection sensitivity increased in the presence of chloride ions and remained constant for 2–24 h with 0.05–0.57 mol L−1 chloride. Therefore, NEDA solution containing chloride ions was used as a chromogenic reagent for the determination of iron(III). The determination range for this method was 0.1–20 mgFe(III) L−1 in a 5 cm glass cell. The developed method is highly selective for iron(III) and has been successfully applied to freshwater, brackish water, seawater, turbid water in rivers, as well as to riverbed and freshwater lake sediments. In addition, a combination of the proposed NEDA method and the 1,10-phenanthroline method enabled simultaneous determination of iron(III) and iron(II).
Graphical abstract











Similar content being viewed by others
Date availability
Data are available based on the request.
References
JISK0101, Test Methods for Industrial Water (Japanese Industrial Standards Committee, Tokyo, 2017), p. 260
JISK0102, Test Methods for Industrial Wastewater (Japanese Industrial Standards Committee, Tokyo, 2016), p. 236
Y. Okura, Bunseki Kagaku 27, 477 (1977) (in Japanese with English abstract). https://doi.org/10.2116/bunsekikagaku.27.8_477
L.L. Stookey, Anal. Chem. 42, 779 (1970). https://epic.awi.de/32905/1/Stookey-1970.pdf
M.M. Gibbs, Water Res. 13, 295 (1979). https://doi.org/10.1016/0043-1354(79)90209-4
M. Okumura, Y. Seike, K. Fu**aga, K. Hirao, Anal. Sci. 13, 231 (1997). https://doi.org/10.2116/analsci.13.231
E.B. Sandell, Colorimetric Determination of Traces of Metals, 3rd edn. (Interscience Publishers Inc., New York, 1959), p.524
I.M. Kolthoff, P.J. Elving, Treatise Anal. Chem. 2, 286 (1962)
K. Hayashi, Y. Sasaki, S. Tagashira, K. Harada, K. Okamura, Bunseki Kagaku 27, 338 (1978) (in Japanese with English abstract). https://doi.org/10.2116/bunsekikagaku.27.6_338
H. Matsuo, M. Chaki, S. Hara, Bunseki Kagaku 15, 692 (1966) (in Japanese with English abstract). https://doi.org/10.2116/bunsekikagaku.15.692
H. Akaiwa, H. Kawamoto, Y. Mimochi, Bunseki Kagaku 30, 310 (1981) (in Japanese with English abstract). https://doi.org/10.2116/bunsekikagaku.30.5_310
M. Yamazaki, I. Mori, K. Enoki, Bunseki Kagaku 21, 897 (1972) (in Japanese with English abstract). https://doi.org/10.2116/bunsekikagaku.21.897
J.P.Riley, G. Skirrows, Chemical Oceanography (Academic Press, New York, 1965), p. 648
A. Hikino, S. Sgahara, T. Kato, Y. Senga, M. Egawa, J.Y., Park, H. Kamiya, H. Tanaka, Y. Seike, Anal. Sci. 37, 347 (2021). https://doi.org/10.2116/analsci.20P254
N. Fujisawa, K. Furubayashi, M. Fukushima, M. Yamamoto, T. Komai, K. Ootsuka, Y. Kawabe, Humic Subst. Res. 8, 1 (2011). https://www.research.kobe-u.ac.jp/ans-soil/jhss/_userdata/hsr8_pp1.pdf
Acknowledgements
Parts of this study were performed with support from the Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Scientific Research (No. 18K14262 to Egawa). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Michiko Egawa, Shogo Sugahara, Keiya Miwa, J.Y. Park, and Yukiko Senga. The first draft of manuscript was written by Michiko Egawa and Yasushi Seike, and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Egawa, M., Sugahara, S., Miwa, K. et al. Development of absorption spectrophotometry of iron(III) in environmental water and sediments using NEDA and its application to the field. ANAL. SCI. (2024). https://doi.org/10.1007/s44211-024-00598-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44211-024-00598-4