Abstract
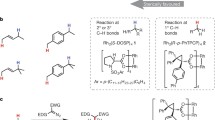
For many years, the development of new methods and catalytic processes for the functionalization of C–H bonds has been among the primary challenges in the field of synthetic chemistry. The selective functionalization of light alkanes offers the possibility of expanded and more efficient use of natural gas, as the functionalization of larger hydrocarbons (e.g., long chain alkanes or arenes) could afford less expensive processes that are more environmentally benign as well as access to entirely new compounds, and the selective C–H functionalization of more complex organic compounds offers strategies for improved routes to prepare high-value fine chemicals.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
BP Statistical Review of World Energy June 2016 (British Petroleum, London, 2016)
Liquefied Natural Gas: Understanding the Basic Facts (U.S. Department of Energy, Washington D.C., 2005)
B.G. Hashiguchi, S.M. Bischof, M.M. Konnick, R.A. Periana, Designing catalysts for functionalization of unactivated C–H bonds based on the CH activation reaction. Acc. Chem. Res. 45, 885–898 (2012)
C.D. Elvidge, M. Zhizhin, K. Baugh, F.C. Hsu, T. Ghosh, Methods for global survey of natural gas flaring from visible infrared imaging radiometer suite data. Energies 9, 1–15 (2016)
Z. Zhu, J.H. Espenson, Organic reactions catalyzed by methylrhenium trioxide: reactions of ethyl diazoacetate and organic azides. J. Am. Chem. Soc. 118, 9901–9907 (1996)
S.J. Blanksby, G.B. Ellison, Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003)
CRC Handbook of Chemistry and Physics, 65th edn (CRC Press, Boca Raton, FL, 1984)
A. Malik, V. Mantri, Gas-to-liquids plants face challenges in the U.S. market, in Today in Energy (U.S. Energy Information Administration: Online, 2014)
J.R. Webb, T. Bolaño, T.B. Gunnoe, Catalytic oxy-functionalization of methane and other hydrocarbons: fundamental advancements and new strategies. ChemSusChem 4, 37–49 (2011)
A.E. Shilov, G.B. Shul’pin, Activation of C–H bonds by metal complexes. Chem. Rev. 97, 2879–2932 (1997)
V.N. Cavaliere, D.J. Mindiola, Methane: a new frontier in organometallic chemistry. Chem. Sci. 3, 3356–3365 (2012)
R.A. Periana, G. Bhalla, W.J. Tenn III, K.J.H. Young, X.Y. Liu, O. Mironov, C.J. Jones, V.R. Ziatdinov, Perspectives on some challenges and approaches for develo** the next generation of selective, low temperature, oxidation catalysts for alkane hydroxylation based on the CH activation reaction. J. Mol. Catal. A: Chem. 220, 7–25 (2004)
D. Klein, Radical Reactions, Organic Chemistry, 2nd edn. (Wiley, Hoboken, NJ, 2015), pp. 500–539
G. Egloff, R.E. Schaad, C.D. Lowry, The halogenation of the paraffin hydrocarbons. Chem. Rev. 8, 1–80 (1931)
R.H. Crabtree, Aspects of methane chemistry. Chem. Rev. 95, 987–1007 (1995)
A.J. Magistro, J.A. Cowfer, Oxychlorination of ethylene. J. Chem. Ed. 63, 1056 (1986)
S.G. Podkolzin, E.E. Stangland, M.E. Jones, E. Peringer, J.A. Lercher, Methyl chloride production from methane over lanthanum-based catalysts. J. Am. Chem. Soc. 129, 2569–2576 (2007)
R.A. Periana, O. Mirinov, D.J. Taube, S. Gamble, High yield conversion of methane to methyl bisulfate catalyzed by iodine cations. Chem. Commun. 2376–2377 (2002)
X. Gang, Y. Zhu, H. Birch, H.A. Hjuler, N.J. Bjerrum, Iodine as catalyst for the direct oxidation of methane to methyl sulfates in oleum. Appl. Catal. A 261, 91–98 (2004)
B. Michalkiewicz, M. Jarosińska, I. Łukasiewicz, Kinetic study on catalytic methane esterification in oleum catalyzed by iodine. Chem. Eng. J. 154, 156–161 (2009)
J. Buddrus, H. Plettenberg, Selective oxidation of alkanes and ethers with iodine tris(trifluoroacetate). Angew. Chem. Int. Ed. 15, 436 (1976)
M.M. Konnick, B.G. Hashiguchi, D. Devarajan, N.C. Boaz, T.B. Gunnoe, J.T. Groves, N. Gunsalus, D.H. Ess, R.A. Periana, Selective CH functionalization of methane, ethane, and propane by a perfluoroarene iodine(III) complex. Angew. Chem. Int. Ed. 53, 10490–10494 (2014)
G.C. Fortman, N.C. Boaz, D. Munz, M.M. Konnick, R.A. Periana, J.T. Groves, T.B. Gunnoe, Selective monooxidation of light alkanes using chloride and iodate. J. Am. Chem. Soc. 136, 8393–8401 (2014)
S.E. Kalman, D. Munz, G.C. Fortman, N.C. Boaz, J.T. Groves, T.B. Gunnoe, Partial oxidation of light alkanes by periodate and chloride salts. Dalton Trans. 44, 5294–5298 (2015)
R.A. Periana, D.J. Taube, S. Gamble, H. Taube, T. Satoh, H. Fujii, Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science 280, 560–564 (1998)
G. Zichittella, V. Paunovic, A.P. Amrute, J. Perez-Ramirez, Catalytic oxychlorination versus oxybromination for methane functionalization. ACS Catal. 7, 1805–1817 (2017)
B. Maillard, K.U. Ingold, J.C. Scaiano, Rate constants for the reactions of free radicals with oxygen in solution. J. Am. Chem. Soc. 105, 5095–5099 (1983)
S.C.W. Hook, B. Saville, The Trap** of carbon radicals. the competition of oxygen and iodine for the 1,1-diphenylethyl radical. J. Chem. Soc. Perkin Trans. 2, 589–593 (1975)
S.P. Mezyk, K.P. Madden, Arrhenius parameter determination for the reaction of methyl radicals with iodine species in aqueous solution. J. Phys. Chem. 100, 9360–9364 (1996)
N.C. Boaz, Mechanistic studies of the iodate mediated monooxidation of light alkanes (2016)
R. Fu, R.J. Nielsen, N.A. Schwartz, W.A. Goddard III, T.B. Gunnoe, J.T. Groves, DFT mechanistic study of methane monooxygenation by hypervalent iodine alkane oxidation (HIAO) process. Manuscript submitted. J. Chem. Phys. C. 123, 15674 (2019)
G.W. Smith, H.D. Williams, Some reactions of adamantane and adamantane derivatives. J. Org. Chem. 26, 2207–2212 (1961)
S.H. Combe, A. Hosseini, A. Parra, P.R. Schreiner, Mild aliphatic and benzylic hydrocarbon C–H bond chlorination using trichloroisocyanuric acid. J. Org. Chem. 82, 2407–2413 (2017)
S. Bloom, C.R. Pitts, D.C. Miller, N. Haselton, M.G. Holl, E. Urheim, T. Lectka, A polycomponent metal-catalyzed aliphatic, allylic, and benzylic fluorination. Angew. Chem. Int. Ed. 51, 10580–10583 (2012)
W. Liu, X. Huang, M.-J. Cheng, R.J. Nielsen, W.A. Goddard III, J.T. Groves, Oxidative aliphatic C–H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science 337, 1322–1325 (2012)
N.C. Boaz, N.A. Schwartz, S.E. Kalman, T.B. Gunnoe, J.T. Groves, Manuscript in preparation. ACS Catal. 8, 3138 (2018)
M. Ahlquist, R.J. Nielsen, R.A. Periana, W.A. Goddard III, Product protection, the key to develo** high performance methane selective oxidation catalysts. J. Am. Chem. Soc. 131, 17110–17715 (2009)
N.F. Goldshleger, A.A. Shteinma, A.E. Shilov, A.E. Shilov, V.V. Eskova, Reactions of alkanes in solutions of chloride complexes of platinum. Russ. J. Phys. Chem. 46, 785–786 (1972)
N.F. Goldshleger, M.B. Tyabin, A.E. Shilov, A.A. Shteinman, Activation of saturated hydrocarbons-deuterium-hydrogen exchange in solutions of transition metal complexes. Russ. J. Phys. Chem. 43, 1222–1223 (1969)
P.J. Pérez, Alkane CH Activation by Single-Site Metal Catalysis (Springer Science & Business Media, vol. 38, 2012)
S.S. Stahl, J.A. Labinger, J.E. Bercaw, Homogeneous oxidation of alkanes by electrophilic late transition metals. Angew. Chem. Int. Ed. 37, 2180–2192 (1998)
R.A. Periana, D.J. Taube, E.R. Evitt, D.G. Löffler, P.R. Wentrcek, G. Voss, T. Masuda, A mercury-catalyzed, high-yield system for the oxidation of methane to methanol. Science 259, 340–343 (1993)
R.A. Periana, O. Mironov, D. Taube, G. Bhalla, C.J. Jones, Catalytic, oxidative condensation of CH4 to CH3COOH in One Step via CH activation. Science 301, 814–818 (2003)
C.J. Jones, D. Taube, V.R. Ziatdinov, R.A. Periana, R.J. Nielsen, J. Oxgaard, W.A. Goddard III, Selective oxidation of methane to methanol catalyzed, with CH activation, by homogeneous, cationic gold. Angew. Chem. 116, 4726–4729 (2004)
O.A. Mironov, S.M. Bischof, M.M. Konnick, B.G. Hashiguchi, V.R. Ziatdinov, W.A. Goddard, M. Ahlquist, R.A. Periana, Using reduced catalysts for oxidation reactions: mechanistic studies of the “Periana-Catalytica” system for CH4 oxidation. J. Am. Chem. Soc. 135, 14644–14658 (2013)
B. Michalkiewicz, J. Ziebro, M. Tomaszewska, Preliminary investigation of low pressure membrane distillation of methyl bisulphate from its solutions in fuming sulphuric acid combined with hydrolysis to methanol. J. Membr. Sci. 286, 223–227 (2006)
R.H. Crabtree, Alkane C–H activation and functionalization with homogeneous transition metal catalysts: a century of progress-a new millennium in prospect. J. Chem. Soc. Dalton Trans. 2437–2450 (2001)
M.-J. Cheng, S.M. Bischof, R.J. Nielsen, W.A. Goddard III, T.B. Gunnoe, R.A. Periana, The para-substituent effect and pH-dependence of the organometallic Baeyer-Villiger oxidation of rhenium-carbon bonds. Dalton Trans. 41, 3758–3763 (2012)
D. Devarajan, T.B. Gunnoe, D.H. Ess, Theory of late-transition-metal alkyl and heteroatom bonding: analysis of Pt, Ru, Ir, and Rh complexes. Inorg. Chem. 51, 6710–6718 (2012)
T.M. Figg, J.R. Webb, T.R. Cundari, T.B. Gunnoe, Carbon-oxygen bond formation via organometallic baeyer-villiger transformations: a computational study on the impact of metal identity. J. Am. Chem. Soc. 134, 2332–2339 (2012)
B.G. Hashiguchi, K.J.H. Young, M. Yousufuddin, W.A. Goddard III, R.A. Periana, Acceleration of nucleophilic CH activation by strongly basic solvents. J. Am. Chem. Soc. 132, 12542–12545 (2010)
J. Kovach, W.W. Brennessel, W.D. Jones, Electrophilic C–H activation of benzene with a shilov-inspired rhodium(III) diimine complex. J. Organomet. Chem. 793, 192–199 (2015)
J.L. Rhinehart, K.A. Manbeck, S.K. Buzak, G.M. Lippa, W.W. Brennessel, K.I. Goldberg, W.D. Jones, Catalytic arene H/D exchange with novel rhodium and iridium complexes. Organometallics 31, 1943–1952 (2012)
S.K. Hanson, D.M. Heinekey, K.I. Goldberg, C–H bond activation by rhodium(I) phenoxide and acetate complexes: mechanism of H–D exchange between arenes and water. Organometallics 27, 1454–1463 (2008)
J.B. Gary, T.J. Carter, M.S. Sanford, Rh(III) pyridinium substituted bipyridine complexes as catalysts for arene H/D exchange. Top. Catal. 55, 565–570 (2012)
B.A. Vaughan, M.S. Webster-Gardiner, T.R. Cundari, T.B. Gunnoe, A rhodium catalyst for single-step styrene production from benzene and ethylene. Science 348, 421–424 (2015)
X.Y. Liu, K.S. Lokare, S.K. Ganesh, J.M. Gonzales, J. Oxgaard, W.A. Goddard Iii, R.A. Periana, Rhodium complexes bearing tetradentate diamine-Bis (phenolate) ligands. Dalton Trans. 40, 301–304 (2011)
M.S. Webster-Gardiner, R. Fu, G.C. Fortman, R.J. Nielsen, T.B. Gunnoe, W.A. Goddard III, Arene C–H activation using Rh(I) catalysts supported by bidentate nitrogen chelates. Catal. Sci. Technol. 5, 96–100 (2015)
H. Chen, S. Schlecht, T.C. Semple, J.F. Hartwig, Thermal, catalytic, regiospecific functionalization of alkanes. Science 287, 1995–1997 (2000)
S.R. Klei, K.L. Tan, J.T. Golden, C.M. Yung, R.K. Thalji, K.A. Ahrendt, J.A. Ellman, T.D. Tilley, R.G. Bergman, in Carbon-Hydrogen Bond Activation by Iridium and Rhodium Complexes: Catalytic Hydrogen/Deuterium Exchange and Carbon-Carbon Bond-Forming Reactions (ACS Symposium Series, 2004)
W.D. Jones, On the nature of carbon–hydrogen bond activation at rhodium and related reactions. Inorg. Chem. 44, 4475–4484 (2005)
B.A. Arndtsen, R.G. Bergman, T.A. Mobley, T.H. Peterson, Selective intermolecular carbon-hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc. Chem. Res. 28, 154–162 (1995)
B.A. Vaughan, S.K. Khani, J.B. Gary, J.D. Kammert, M.S. Webster-Gardiner, B.A. McKeown, R.J. Davis, T.R. Cundari, T.B. Gunnoe, Mechanistic studies of single-step styrene production using a rhodium(I) catalyst. J. Am. Chem. Soc. 139, 1485–1498 (2017)
M.S. Webster-Gardiner, J. Chen, B.A. Vaughan, B.A. McKeown, W. Schinski, T.B. Gunnoe, Catalytic synthesis of “Super” linear alkenyl arenes using an easily prepared Rh(I) catalyst. J. Am. Chem. Soc. 139, 5474–5480 (2017)
D.H. Ess, T.B. Gunnoe, T.R. Cundari, W.A. Goddard, R.A. Periana, Ligand lone-pair influence on hydrocarbon C–H activation: a computational perspective. Organometallics 29, 6801–6815 (2010)
S.R. Golisz, T.B. Gunnoe, W.A. Goddard III, J.T. Groves, R.A. Periana, Chemistry in the center for catalytic hydrocarbon functionalization: an energy Frontier Research Center. Catal. Lett. 141, 213–221 (2011)
S.M. Bischof, M.-J. Cheng, R.J. Nielsen, T.B. Gunnoe, W.A. Goddard III, R.A. Periana, Functionalization of rhenium aryl bonds by O-atom transfer. Organometallics 30, 2079–2082 (2011)
M.E. O’Reilly, R. Fu, R.J. Nielsen, M. Sabat, W.A. Goddard III, T.B. Gunnoe, Long-range C–H bond activation by RhIII-carboxylates. J. Am. Chem. Soc. 136, 14690–14693 (2014)
R. Fu, R.J. Nielsen, W.A. Goddard, G.C. Fortman, T.B. Gunnoe, DFT virtual screening identifies rhodium-amidinate complexes as potential homogeneous catalysts for methane-to-methanol oxidation. ACS Catal. 4, 4455–4465 (2014)
R. Fu, M.E. O’Reilly, R.J. Nielsen, W.A. Goddard III, T.B. Gunnoe, Rhodium Bis (quinolinyl)benzene complexes for methane activation and functionalization. Chem. Eur. J. 21, 1286–1293 (2015)
D. Munz, M.S. Webster-Gardiner, R. Fu, T. Strassner, W.A. Goddard, T.B. Gunnoe, Proton or metal? The H/D exchange of arenes in acidic solvents. ACS Catal. 5, 769–775 (2015)
M.E. O’Reilly, S.I. Johnson, R.J. Nielsen, W.A. Goddard III, T.B. Gunnoe, Transition-metal-mediated nucleophilic aromatic substitution with acids. Organometallics 35, 2053–2056 (2016)
M.S. Webster-Gardiner, P.E. Piszel, R. Fu, B.A. McKeown, R.J. Nielsen, W.A. Goddard III, T.B. Gunnoe, Electrophilic RhI catalysts for arene H/D exchange in acidic media: evidence for an electrophilic aromatic substitution mechanism. J. Mol. Catal. A: Chem. 426, 381–388 (2017)
A.J. Hickman, J.M. Villalobos, M.S. Sanford, Quantitative assay for the direct comparison of platinum catalysts in benzene H/D exchange. Organometallics 28, 5316–5322 (2009)
M.H. Emmert, J.B. Gary, J.M. Villalobos, M.S. Sanford, Platinum and palladium complexes containing cationic ligands as catalysts for arene H/D exchange and oxidation. Angew. Chem. Int. Ed. 49, 5884–5886 (2010)
M.C. Lehman, J.B. Gary, P.D. Boyle, M.S. Sanford, E.A. Ison, Effect of solvent and ancillary ligands on the catalytic H/D exchange reactivity of Cp*IrIII(L) complexes. ACS Catal. 3, 2304–2310 (2013)
N.A. Isley, R.T.H. Linstadt, S.M. Kelly, F. Gallou, B.H. Lipshutz, Nucleophilic aromatic substitution reactions in water enabled by micellar catalysis. Org. Lett. 17, 4734–4737 (2015)
J.F. Bunnett, R.E. Zahler, Aromatic nucleophilic substitution reactions. Chem. Rev. 49, 273–412 (1951)
J.R. Rodriguez, J. Agejas, A.B. Bueno, Practical synthesis of aromatic ethers by SNAr of fluorobenzenes with alkoxides. Tetrahedron Lett. 47, 5661–5663 (2006)
G. Bartoli, M. Bosco, A. Carlone, M. Locatelli, E. Marcantoni, P. Melchiorre, L. Sambri, tert-butyl ethers: renaissance of an alcohol protecting group. Facile cleavage with cerium(III) chloride/sodium iodide. Adv. Synth. Catal. 348, 905–910 (2006)
A. Procopio, P. Costanzo, M. Curini, M. Nardi, M. Oliverio, R. Paonessa, An eco-sustainable erbium (III) triflate catalyzed formation and cleavage of tert-butyl ethers. Synthesis 2011, 73–78
M.E. O’Reilly, D.R. Pahls, J.R. Webb, N.C. Boaz, S. Majumdar, C.D. Hoff, J.T. Groves, T.R. Cundari, T.B. Gunnoe, Reductive functionalization of a rhodium(III)-methyl bond by electronic modification of the supporting ligand. Dalton Trans. 43, 8273–8281 (2014)
M.E. O’Reilly, D.R. Pahls, T.R. Cundari, T.B. Gunnoe, Reductive functionalization of a rhodium(III)–methyl bond in acidic media: key step in the electrophilic functionalization of methane. Organometallics 33, 6504–6510 (2014)
J.F. Hartwig, Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 138, 2–24 (2016)
J.A. Labinger, J.E. Bercaw, Understanding and exploiting C-H bond activation. Nature 417, 507–514 (2002)
D.A. Colby, R.G. Bergman, J.A. Ellman, Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–655 (2010)
T.W. Lyons, M.S. Sanford, Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010)
A. Studer, D.P. Curran, Catalysis of radical reactions: a radical chemistry perspective. Angew. Chem. Int. Ed. 55, 58–102 (2016)
X. Huang, J.T. Groves, Taming azide radicals for catalytic C–H azidation. ACS Catal. 6, 751–759 (2016)
M. Rossberg, W. Lendle, G. Pfleiderer, A. Tögel, E.-L. Dreher, E. Langer, H. Rassaerts, P. Kleinschmidt, H. Strack, R. Cook, U. Beck, K.-A. Lipper, T.R. Torkelson, E. Löser, K.K. Beutel, T. Mann, Chlorinated Hydrocarbons, in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH Weinheim, 2006)
C.K. Prier, D.A. Rankic, D.W.C. MacMillan, Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013)
J. **e, H. **, P. Xu, C. Zhu, When C-H bond functionalization meets visible-light photoredox catalysis. Tetrahedron Lett. 55, 36–48 (2014)
X. Huang, J.T. Groves, Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C–H activation. J. Biol. Inorg. Chem. 22, 185–207 (2017)
X. Wang, R. Ullrich, M. Hofrichter, J.T. Groves, Heme-thiolate ferryl of aromatic peroxygenase is basic and reactive. Proc. Natl. Acad. Sci. U.S.A. 112, 3686–3691 (2015)
R.J. Martinie, J. Livada, W.C. Chang, M.T. Green, C. Krebs, J.M. Bollinger, A. Silakov, Experimental correlation of substrate position with reaction outcome in the aliphatic halogenase, SyrB2. J. Am. Chem. Soc. 137, 6912–6919 (2015)
M.L. Matthews, C.S. Neumann, L.A. Miles, T.L. Grove, S.J. Booker, C. Krebs, C.T. Walsh, J.M. Bollinger, Substrate positioning controls the partition between halogenation and hydroxylation in the aliphatic halogenase, SyrB2. Proc. Natl. Acad. Sci. U.S.A. 106, 17723–17728 (2009)
C.-M. Che, V.K.-Y. Lo, C.-Y. Zhou, J.-S. Huang, Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011)
I. Bauer, H.-J. Knoelker, Iron catalysis in organic synthesis. Chem. Rev. 115, 3170–3387 (2015)
J.T. Groves, W.J. Kruper, R.C. Haushalter, Hydrocarbon oxidations with oxometalloporphinates. Isolation and reactions of a (Porphinato) manganese(V) complex. J. Am. Chem. Soc. 102, 6375–6377 (1980)
C.L. Hill, J.A. Smegal, T.J. Henly, Catalytic replacement of unactivated alkane carbon-hydrogen bonds with carbon-X bonds (X = nitrogen, oxygen, chlorine, bromine, or iodine). Coupling of intermolecular hydrocarbon activation by MnIIITPPX Complexes With Phase-Transfer Catalysis. J. Org. Chem. 48, 3277–3281 (1983)
T. Kojima, R.A. Leising, S.P. Yan, L. Que, Alkane functionalization at nonheme iron centers. Stoichiometric transfer of metal-bound ligands to alkane. J. Am. Chem. Soc. 115, 11328–11335 (1993)
M. Puri, A.N. Biswas, R. Fan, Y. Guo, L. Que, Modeling non-heme iron halogenases: high-spin oxoiron(IV)–halide complexes that halogenate C–H bonds. J. Am. Chem. Soc. 138, 2484 (2016)
O. Planas, M. Clemancey, J.C. Latour, A. Company, M. Costas, Structural modeling of iron halogenases: synthesis and reactivity of halide-iron(IV)-oxo compounds. Chem. Commun. 50, 10887–10890 (2014)
W. Liu, J.T. Groves, Manganese porphyrins catalyze selective C–H bond halogenations. J. Am. Chem. Soc. 132, 12847–12849 (2010)
W. Liu, J.T. Groves, Manganese catalyzed C–H halogenation. Acc. Chem. Res. 48, 1727–1735 (2015)
W. Liu, M.J. Cheng, R.J. Nielsen, W.A. Goddard III, J.T. Groves, Probing the C–O bond-formation step in metalloporphyrin catalyzed C–H oxygenation reactions. ACS Catal. 7, 4182–4188 (2017)
M. Rueda-Becerril, C.C. Sazepin, J.C.T. Leung, T. Okbinoglu, P. Kennepohl, J.F. Paquin, G.M. Sammis, Fluorine transfer to alkyl radicals. J. Am. Chem. Soc. 134, 4026–4029 (2012)
C.R. Pitts, S. Bloom, R. Woltornist, D.J. Auvenshine, L.R. Ryzhkov, M.A. Siegler, T. Lectka, Direct, catalytic monofluorination of sp3 C–H bonds: a radical-based mechanism with ionic selectivity. J. Am. Chem. Soc. 136, 9780–9791 (2014)
J.-B. **a, C. Zhu, C. Chen, Visible light-promoted metal-Free C–H activation: diarylketone-catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc. 135, 17494–17500 (2013)
Y. Amaoka, M. Nagatomo, M. Inoue, Metal-free fluorination of C(sp3)-H bonds using a catalytic N-oxyl radical. Org. Lett. 15, 2160–2163 (2013)
D. O’Hagan, Understanding organofluorine chemistry. an introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008)
T. Furuya, A.S. Kamlet, T. Ritter, Catalysis for fluorination and trifluoromethylation. Nature 473, 470–477 (2011)
C. Hollingworth, V. Gouverneur, Transition metal catalysis and nucleophilic fluorination. Chem. Commun. 48, 2929–2942 (2012)
X. Huang, W. Liu, H. Ren, R. Neelamegam, J.M. Hooker, J.T. Groves, Late stage benzylic C-H fluorination with [18F]Fluoride for PET imaging. J. Am. Chem. Soc. 136, 6842–6845 (2014)
X.Y. Huang, T.M. Bergsten, J.T. Groves, Manganese-catalyzed late-stage aliphatic C-H azidation. J. Am. Chem. Soc. 137, 5300–5303 (2015)
P. Thirumurugan, D. Matosiuk, K. Jozwiak, Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 113, 4905–4979 (2013)
J.K. Kochi, Electron-transfer mechanisms for organometallic intermediates in catalytic reactions. Acc. Chem. Res. 7, 351–360 (1974)
C.L. Jenkins, J.K. Kochi, Ligand transfer of halides (Cl, Br, I) and pseudohalides (SCN, N3, CN) from copper(II) to alkyl radicals. 1. J. Org. Chem. 36, 3095–3102 (1971)
J.K. Kochi, Mechanisms of organic oxidation and reduction by metal complexes. Science 155, 415–424 (1967)
Z. Zuo, D.T. Ahneman, L. Chu, J.A. Terrett, A.G. Doyle, D.W.C. MacMillan, Merging photoredox with nickel catalysis: coupling of alpha-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014)
A. Noble, S.J. McCarver, D.W.C. MacMillan, Merging photoredox and nickel catalysis: decarboxylative cross-coupling of carboxylic acids with vinyl halides. J. Am. Chem. Soc. 137, 624–627 (2015)
J.C. Tellis, D.N. Primer, G.A. Molander, Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 345, 433–436 (2014)
Q.M. Kainz, C.D. Matier, A. Bartoszewicz, S.L. Zultanski, J.C. Peters, G.C. Fu, Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 351, 681–684 (2016)
E.B. Corcoran, M.T. Pirnot, S. Lin, S.D. Dreher, D.A. DiRocco, I.W. Davies, S.L. Buchwald, D.W.C. MacMillan, Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 353, 279–283 (2016)
Y. Ye, M.S. Sanford, Merging visible-light photocatalysis and transition-metal catalysis in the copper-catalyzed trifluoromethylation of boronic acids with CF3I. J. Am. Chem. Soc. 134, 9034–9037 (2012)
M.D. Levin, S. Kim, F.D. Toste, Photoredox catalysis unlocks single-electron elementary steps in transition metal catalyzed cross-coupling. ACS Cent. Sci. 2, 293–301 (2016)
J. Wang, T. Qin, T.-G. Chen, L. Wimmer, J.T. Edwards, J. Cornella, B. Vokits, S.A. Shaw, P.S. Baran, Nickel-catalyzed cross-coupling of redox-active esters with boronic acids. Angew. Chem. Int. Ed. 55, 9676–9679 (2016)
Acknowledgement and Dedication
This contribution is provided to celebrate the many contributions of William A. Goddard III, whose remarkable career has contributed fundamental advancements across an impressive array of disciplines. More importantly, Bill’s impact as a mentor has positively impacted hundreds of individuals who have benefitted from his knowledge, creativity and enthusiasm. It has been a distinct pleasure to collaborate scientifically with Bill, and we consider ourselves fortunate to count him among our friends. TBG acknowledges support from the U.S. Department of Energy, Office of Basic Energy Sciences (DE-SC0000776), and the National Science Foundation (CHE-1465145). JTG acknowledges support from the U.S. National Science Foundation (CHE-1148597 and CHE-1464578).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Liebov, N.S. et al. (2021). Studies of C–H Activation and Functionalization: Combined Computational and Experimental Efforts to Elucidate Mechanisms, Principles, and Catalysts. In: Shankar, S., Muller, R., Dunning, T., Chen, G.H. (eds) Computational Materials, Chemistry, and Biochemistry: From Bold Initiatives to the Last Mile. Springer Series in Materials Science, vol 284. Springer, Cham. https://doi.org/10.1007/978-3-030-18778-1_34
Download citation
DOI: https://doi.org/10.1007/978-3-030-18778-1_34
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18777-4
Online ISBN: 978-3-030-18778-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)