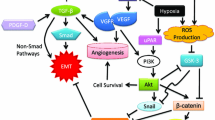
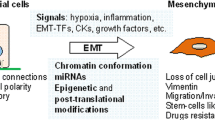
Abstract
In this chapter you will learn that, besides the initial oncogenic mutations that trigger tumorigenesis, cancer cells must have additional molecular changes and morphological modifications in order to metastasize and become malignant. One of the mechanisms that tumor cells may undergo to achieve this state is the Epithelial-Mesenchymal Transition (EMT). After giving a global perspective of what EMT is and of the main EMT activating factors, the particularities of EMT in tumors and the molecular mechanisms that underlie the tumor EMT phenotypic plasticity are adressed. Finally, the contribution of EMT studies for the development of a new generation of cancer therapies is discussed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127(4):679–695. https://doi.org/10.1016/J.CELL.2006.11.001
Nguyen DX, Bos PD, Massagué J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–284. https://doi.org/10.1038/nrc2622
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147(2):275–292. https://doi.org/10.1016/j.cell.2011.09.024
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol 17(5):548–558. https://doi.org/10.1016/J.CEB.2005.08.001
Meng F, Wu G (2012) The rejuvenated scenario of epithelial--mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev 31(3):455–467. https://doi.org/10.1007/s10555-012-9379-3
Mayor R, Carmona-Fontaine C (2010) Kee** in touch with contact inhibition of locomotion. Trends Cell Biol 20(6):319–328. https://doi.org/10.1016/j.tcb.2010.03.005
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572. https://doi.org/10.1038/nrc865
Micalizzi DS, Farabaugh SM, Ford HL (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15(2):117–134. https://doi.org/10.1007/s10911-010-9178-9
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. https://doi.org/10.1016/J.CELL.2011.02.013
Powell DR, Blasky AJ, Britt SG, Artinger KB (2013) Riding the crest of the wave: parallels between the neural crest and cancer in epithelial-to-mesenchymal transition and migration. Wiley Interdiscip Rev Syst Biol Med 5(4):511–522. https://doi.org/10.1002/wsbm.1224
Chaffer CL, Thompson EW, Williams ED (2007) Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 185(1–3):7–19. https://doi.org/10.1159/000101298
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559 LP–1551564. http://science.sciencemag.org/content/331/6024/1559.abstract
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454. https://doi.org/10.1038/nrc822
Hay ED (1968) Organization and fine structure of epithelium and mesenchyme in the develo** chick embryo. In: Fleischmajer R, Billingham RE (eds) Epithel. Baltimore, MD, USA, Williams & Wilkins Co
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Natl Rev Mol Cell Biol 15(3):178–196. https://doi.org/10.1038/nrm3758.Molecular
Nieto MA (2013) Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342:6159. http://science.sciencemag.org/content/342/6159/1234850.abstract
Qin Y, Capaldo C, Gumbiner BM, Macara IG (2005) The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171(6):1061 LP–1061071. http://jcb.rupress.org/content/171/6/1061.abstract
Whiteman EL, Liu C-J, Fearon ER, Margolis B (2008) The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 27:3875–3879. https://doi.org/10.1038/onc.2008.9
Huang RY-J, Guilford P, Thiery JP (2012) Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci 125(19):4417 LP–4414422. http://jcs.biologists.org/content/125/19/4417.abstract
Kalluri R, Weinberg RA (2009) Review series The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428. https://doi.org/10.1172/JCI39104.1420
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142. https://doi.org/10.1038/nrm1835
Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR (2008) Cadherin switching. J Cell Sci 121(6):727 LP–727735. http://jcs.biologists.org/content/121/6/727.abstract
Beaty BT, Condeelis J (2014) Digging a little deeper: The stages of invadopodium formation and maturation. Eur J Cell Biol 93(10–12):438–444. https://doi.org/10.1016/J.EJCB.2014.07.003
Leong HS, Robertson AE, Stoletov K et al (2014) Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep 8(5):1558–1570. https://doi.org/10.1016/j.celrep.2014.07.050
Nistico P, Bissell MJ, Radisky DC (2012) Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol 4(2):a011908–a011908. https://doi.org/10.1101/cshperspect.a011908
Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172(7):973 LP–973981. http://jcb.rupress.org/content/172/7/973.abstract
Kuriyama S, Mayor R (2008) Molecular analysis of neural crest migration. Philos Trans R Soc B Biol Sci 363(1495):1349 LP–1341362. http://rstb.royalsocietypublishing.org/content/363/1495/1349.abstract
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119(6):1438–1449. https://doi.org/10.1172/JCI38019
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. https://doi.org/10.1016/j.cell.2009.11.007
Nieto MA, Cano A (2012) The epithelial–mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol 22(5–6):361–368. https://doi.org/10.1016/J.SEMCANCER.2012.05.003
Nieto MA, Sargent MG, Wilkinson DG, Cooke J (1994) Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264(5160):835 LP–835839. http://science.sciencemag.org/content/264/5160/835.abstract
Theveneau E, Mayor R (2012) Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol 366(1):34–54. https://doi.org/10.1016/j.ydbio.2011.12.041
Lindsey S, Langhans SA (2014) Crosstalk of oncogenic signaling pathways during epithelial–mesenchymal transition. Front Oncol 4:358. https://www.frontiersin.org/article/10.3389/fonc.2014.00358
Gonzalez DM, Medici D (2014) Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7(344):re8 LP–re8re8. http://stke.sciencemag.org/content/7/344/re8.abstract
Vincent T, Neve EPA, Johnson JR et al (2009) A SNAIL1–SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat Cell Biol 11:943–950. https://doi.org/10.1038/ncb1905
Peinado H, Quintanilla M, Cano A (2003) Transforming growth factor β-1 induces Snail transcription factor in epithelial cell lines. J Biol Chem 278(23):21113–21123. https://doi.org/10.1074/JBC.M211304200
Yang Y, Ahn Y-H, Gibbons DL et al (2011) The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200–dependent pathway in mice. J Clin Invest 121(4):1373–1385. https://doi.org/10.1172/JCI42579
Puisieux A, Brabletz T, Caramel J (2014) Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 16(6). https://doi.org/10.1038/ncb2976
Nawshad A, Hay ED (2003) TGFβ3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J Cell Biol 163(6):1291 LP–1291301. http://jcb.rupress.org/content/163/6/1291.abstract
Batlle E, Sancho E, Francí C et al (2000) The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89. https://doi.org/10.1038/35000034
Eger A, Aigner K, Sonderegger S et al (2005) DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24:2375–2385. https://doi.org/10.1038/sj.onc.1208429
Yang J, Mani SA, Donaher JL et al (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7):927–939. https://doi.org/10.1016/j.cell.2004.06.006
Fang X, Cai Y, Liu J et al (2011) Twist2 contributes to breast cancer progression by promoting an epithelial–mesenchymal transition and cancer stem-like cell self-renewal. Oncogene 30:4707–4720. https://doi.org/10.1038/onc.2011.181
Yang M-H, Hsu DS-S, Wang H-W et al (2010) Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nat Cell Biol 12:982–992. https://doi.org/10.1038/ncb2099
Navarro P, Lozano E, Cano A (1993) Expression of E- or P-cadherin is not sufficient to modify the morphology and the tumorigenic behavior of murine spindle carcinoma cells. Possible involvement of plakoglobin. J Cell Sci 105(4):923 LP–923934. http://jcs.biologists.org/content/105/4/923.abstract
Miyoshi A, Kitajima Y, Sumi K et al (2004) Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer 90:1265–1273. https://doi.org/10.1038/sj.bjc.6601685
Ikenouchi J, Matsuda M, Furuse M, Tsukita S (2003) Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116(10):1959 LP–1951967. http://jcs.biologists.org/content/116/10/1959.abstract
Ohkubo T, Ozawa M (2004) The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci 117(9):1675 LP–1671685. http://jcs.biologists.org/content/117/9/1675.abstract
Zheng H, Kang Y (2013) Multilayer control of the EMT master regulators. Oncogene 33:1755–1763. https://doi.org/10.1038/onc.2013.128
De Craene B, Berx G (2013) Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 13:97–110. https://doi.org/10.1038/nrc3447
Nakaya Y, Sheng G (2008) Epithelial to mesenchymal transition during gastrulation: An embryological view. Develop Growth Differ 50(9):755–766. https://doi.org/10.1111/j.1440-169X.2008.01070.x
Nakaya Y, Sheng G (2009) An amicable separation: chick’s way of doing EMT. Cell Adhes Migr 3(2):160–163. https://www.ncbi.nlm.nih.gov/pubmed/19262172
Hardy KM, Yatskievych TA, Konieczka JH, Bobbs AS, Antin PB (2011) FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev Biol 11(1):20. https://doi.org/10.1186/1471-213X-11-20
Ferrer-Vaquer A, Viotti M, Hadjantonakis A-K (2010) Transitions between epithelial and mesenchymal states and the morphogenesis of the early mouse embryo. Cell Adhes Migr 4(3):447–457. https://doi.org/10.4161/cam.4.3.10771
Le Douarin NM, Dupin E (2018) The “beginnings” of the neural crest. Dev Biol. https://doi.org/10.1016/j.ydbio.2018.07.019
Nakaya Y, Sheng G (2013) EMT in developmental morphogenesis. Cancer Lett 341(1):9–15. https://doi.org/10.1016/J.CANLET.2013.02.037
López-Nouoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1(6–7):303–314. https://doi.org/10.1002/emmm.200900043
Savagner P (2001) Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. BioEssays 23(10):912–923. https://doi.org/10.1002/bies.1132
Savagner P, Kusewitt DF, Carver EA et al (2004) Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol 202(3):858–866. https://doi.org/10.1002/jcp.20188
Shirley SH, Hudson LG, He J, Kusewitt DF (2010) The skinny on slug. Mol Carcinog 49(10):851–861. https://doi.org/10.1002/mc.20674
Desmoulière A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146(1):56–66. https://www.ncbi.nlm.nih.gov/pubmed/7856739
Klingberg F, Hinz B, White ES (2013) The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol 229(2):298–309. https://doi.org/10.1002/path.4104
Fisher R, Pusztai L, Swanton C (2013) Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer 108:479–485. https://doi.org/10.1038/bjc.2012.581
McGranahan N, Swanton C (2017) Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168(4):613–628. https://doi.org/10.1016/j.cell.2017.01.018
Huang RY-J, Wong MK, Tan TZ et al (2013) An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis 4:e915. https://doi.org/10.1038/cddis.2013.442
Nieto MA, Huang RYYJ, Jackson RAA, Thiery JPP (2016) Emt: 2016. Cell 166(1):21–45. https://doi.org/10.1016/j.cell.2016.06.028
Cano A, Pérez-Moreno MA, Rodrigo I et al (2000) The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83. https://doi.org/10.1038/35000025
Tam WL, Weinberg RA (2013) The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 19(11):1438–1449. https://doi.org/10.1038/nm.3336
Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14(6):818–829. https://doi.org/10.1016/j.devcel.2008.05.009
Biddle A, Liang X, Gammon L et al (2011) Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res 71(15):5317 LP–5315326. http://cancerres.aacrjournals.org/content/71/15/5317.abstract
Roesch A, Fukunaga-Kalabis M, Schmidt EC et al (2010) A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 141(4):583–594. https://doi.org/10.1016/J.CELL.2010.04.020
O’Brien-Ball C, Biddle A (2017) Reprogramming to developmental plasticity in cancer stem cells. Dev Biol 430(2):266–274. https://doi.org/10.1016/j.ydbio.2017.07.025
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374. https://doi.org/10.1038/nrc1075
Friedl P, Locker J, Sahai E, Segall JE (2012) Classifying collective cancer cell invasion. Nat Cell Biol 14:777–783. https://doi.org/10.1038/ncb2548
Cheung KJ, Ewald AJ (2016) A collective route to metastasis: seeding by tumor cell clusters. Science 352(6282). https://doi.org/10.1126/science.aaf6546
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(23):5591 LP–5595596. http://jcs.biologists.org/content/125/23/5591.abstract
Chang Q, Bournazou E, Sansone P et al (2013) The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 15(7):848–862. https://www.ncbi.nlm.nih.gov/pubmed/23814496
Robinson BD, Sica GL, Liu Y-F et al (2009) Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res 15(7):2433 LP–2432441. http://clincancerres.aacrjournals.org/content/15/7/2433.abstract
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1):52–67. https://doi.org/10.1016/j.cell.2010.03.015
Chatterjee S, Seifried L, Feigin ME et al (2012) Dysregulation of cell polarity proteins synergize with oncogenes or the microenvironment to induce invasive behavior in epithelial cells. Schneider-Stock R, ed. PLoS One 7(4):e34343. https://doi.org/10.1371/journal.pone.0034343
Hu X, Li D, Zhang W, Zhou J, Tang B, Li L (2012) Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch Gynecol Obstet 286(6):1537–1543. https://doi.org/10.1007/s00404-012-2456-6
Wang J, Ye C, Lu D et al (2017) Matrix metalloproteinase-1 expression in breast carcinoma: a marker for unfavorable prognosis. Oncotarget 8(53):91379–91390. https://doi.org/10.18632/oncotarget.20557
Inoue T, Yashiro M, Nishimura S, Maeda K, Sawada T, Ogawa Y, Sowa MCK (1999) Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int J Mol Med 4(1):73–77
Zucker SVJ (2004) Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 23(1–2):101–117
Noe V, Fingleton B, Jacobs K et al (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114(1):111 LP–111118. http://jcs.biologists.org/content/114/1/111.abstract
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8(2):98–101
Lu X, Kang Y (2007) Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia 12(2):153–162. https://doi.org/10.1007/s10911-007-9047-3
Kaplan RN, Rafii S, Lyden D (2006) Preparing the “soil”: the premetastatic niche. Cancer Res 66(23):11089–11093. https://doi.org/10.1158/0008-5472.CAN-06-2407
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293. https://doi.org/10.1038/nrc2621
Peinado H, Alečković M, Lavotshkin S et al (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18:883–891. https://doi.org/10.1038/nm.2753
Peinado H, Zhang H, Matei IR et al (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17(5). https://doi.org/10.1038/nrc.2017.6
Hoshino A, Costa-Silva B, Shen T-L et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335. https://doi.org/10.1038/nature15756
Gupta GP, Nguyen DX, Chiang AC et al (2007) Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446:765–770. https://doi.org/10.1038/nature05760
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370. https://doi.org/10.1038/nm.2537
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4). https://doi.org/10.1016/j.cell.2016.11.037
Brabletz T (2012) To differentiate or not–routes towards metastasis. Nat Rev Cancer 12(6):425–436. https://doi.org/10.1038/nrc3265. Review. PubMed PMID: 22576165
Hugo H, Ackland ML, Blick T et al (2007) Epithelial - Mesenchymal and mesenchymal - Epithelial transitions in carcinoma progression. J Cell Physiol 213(2):374–383. https://doi.org/10.1002/jcp.21223
Yao D, Dai C, Peng S (2011) Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 9(12):1608–1620. https://doi.org/10.1158/1541-7786.MCR-10-0568
Gao D, Joshi N, Choi H et al (2012) Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res 72(6):1384 LP–1381394. http://cancerres.aacrjournals.org/content/72/6/1384.abstract
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9(1):179. https://doi.org/10.1186/1476-4598-9-179
Jia D, Jolly MK, Kulkarni P, Levine H (2017) Phenotypic plasticity and cell fate decisions in cancer: Insights from dynamical systems theory. Cancers (Basel). 9(7):1–19. https://doi.org/10.3390/cancers9070070
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J (2012) Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22(6):725–736. https://doi.org/10.1016/j.ccr.2012.09.022
Beerling E, Seinstra D, de Wit E et al (2016) Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep 14(10). https://doi.org/10.1016/j.celrep.2016.02.034
Diepenbruck M, Christofori G (2016) Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 43:7–13. https://doi.org/10.1016/J.CEB.2016.06.002
Zheng X, Carstens JL, Kim J et al (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527(7579). https://doi.org/10.1038/nature16064
Fischer KR, Durrans A, Lee S et al (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527(7579). https://doi.org/10.1038/nature15748
Soucheray M, Capelletti M, Pulido I et al (2015) Intratumoral heterogeneity in EGFR-mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res 75(20):4372 LP–4374383. http://cancerres.aacrjournals.org/content/75/20/4372.abstract
Baccelli I, Schneeweiss A, Riethdorf S et al (2013) Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31:539–544. https://doi.org/10.1038/nbt.2576
Tran HD, Luitel K, Kim M, Zhang K, Longmore GD, Tran DD (2014) Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res 74(21):6330 LP–6336340. http://cancerres.aacrjournals.org/content/74/21/6330.abstract
Krebs AM, Mitschke J, Lasierra Losada M et al (2017) The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 19:518–529. https://doi.org/10.1038/ncb3513
Caramel J, Papadogeorgakis E, Hill L et al (2013) A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 24(4):466–480. https://doi.org/10.1016/J.CCR.2013.08.018
Denecker G, Vandamme N, Akay Ö et al (2014) Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ 21:1250–1261. https://doi.org/10.1038/cdd.2014.44
Biddle A, Mackenzie IC (2012) Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev 31(1):285–293. https://doi.org/10.1007/s10555-012-9345-0
Wellner U, Brabletz T, Keck T (2010) ZEB1 in pancreatic cancer. Cancers (Basel) 2(3):1617–1628. https://doi.org/10.3390/cancers2031617
Kurrey NK, Jalgaonkar SP, Joglekar AV et al (2009) Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27(9):2059–2068. https://doi.org/10.1002/stem.154
Visvader JE, Lindeman GJ (2012) Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10(6):717–728. https://doi.org/10.1016/J.STEM.2012.05.007
Lobo NA, Shimono Y, Qian D, Clarke MF (2007) The biology of cancer stem cells. Annu Rev Cell Dev Biol 23(1):675–699. https://doi.org/10.1146/annurev.cellbio.22.010305.104154
Atena M, Reza AM, Mehran G (2014) A review on the biology of cancer stem cells. Stem Cell Discov 04(04):83–89. https://doi.org/10.4236/scd.2014.44009
D’Andrea V, Panarese A, Tonda M, Biffoni MMM (2017) Cancer stem cells as functional biomarkers. Cancer Biomark 20(3):231–234. https://doi.org/10.3233/CBM-151176
Yang M-H, Imrali A, Heeschen C (2015) Circulating cancer stem cells: the importance to select. Chin J Cancer Res 27(5):437–449. https://doi.org/10.3978/j.issn.1000-9604.2015.04.08
Kahlert UD, Joseph JV, Kruyt FAE (2017) EMT- and MET-related processes in nonepithelial tumors: importance for disease progression, prognosis, and therapeutic opportunities. Mol Oncol 11(7):860–877. https://doi.org/10.1002/1878-0261.12085
Sarkar S, Horn G, Moulton K et al (2013) Cancer development, progression, and therapy: an epigenetic overview. Int J Mol Sci 14(10):21087–21113. https://doi.org/10.3390/ijms141021087
Pencheva N, Tavazoie SF (2013) Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol 15(6):546–554. https://doi.org/10.1038/ncb2769
Xu Q, Deng F, Qin Y et al (2016) Long non-coding RNA regulation of epithelial–mesenchymal transition in cancer metastasis. Cell Death Dis 7:e2254. https://doi.org/10.1038/cddis.2016.149
Biamonti G, Bonomi S, Gallo S, Ghigna C (2012) Making alternative splicing decisions during epithelial-to-mesenchymal transition (EMT). Cell Mol Life Sci 69(15):2515–2526. https://doi.org/10.1007/s00018-012-0931-7
Díaz VM, Viñas-Castells R, García de Herreros A (2014) Regulation of the protein stability of EMT transcription factors. Cell Adhes Migr 8(4):418–428. https://doi.org/10.4161/19336918.2014.969998
Jones PA, Baylin SB (2007) The epigenomics of cancer. Cell 128(4):683–692. https://doi.org/10.1016/J.CELL.2007.01.029
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358(11):1148–1159. https://doi.org/10.1056/NEJMra072067
Lujambio A, Ropero S, Ballestar E et al (2007) Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res 67(4):1424 LP–1421429. http://cancerres.aacrjournals.org/content/67/4/1424.abstract
Matsumura N, Huang Z, Mori S et al (2011) Epigenetic suppression of the TGF-beta pathway revealed by transcriptome profiling in ovarian cancer. Genome Res 21(1):74–82. https://doi.org/10.1101/gr.108803.110
Dong Z, Guo W, Guo Y, Kuang G, Yang Z (2012) Concordant promoter methylation of transforming growth factor-beta receptor types I and II occurs early in esophageal squamous cell carcinoma. Am J Med Sci 343(5):375–381. https://doi.org/10.1097/MAJ.0B013E3182253430
Einav Nili G-Y, Saito Y, Egger G, Jones PA (2008) Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med 59(1):267–280. https://doi.org/10.1146/annurev.med.59.061606.095816
Skrypek N, Goossens S, De Smedt E, Vandamme N, Berx G (2017) Epithelial-to-mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends Genet 33(12):943–959. https://doi.org/10.1016/j.tig.2017.08.004
Song SJ, Poliseno L, Song MS et al (2013) MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154(2):311–324. https://doi.org/10.1016/j.cell.2013.06.026
Ma L, Teruya-Feldstein J, Weinberg RA (2007) Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449:682–688. https://doi.org/10.1038/nature06174
He L, Thomson JM, Hemann MT et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833. https://doi.org/10.1038/nature03552
Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K (2010) Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell 39(5):761–772. https://doi.org/10.1016/j.molcel.2010.08.013
Olson P, Lu J, Zhang H et al (2009) MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev 23(18):2152–2165. http://genesdev.cshlp.org/content/23/18/2152.abstract
Breving K, Esquela-Kerscher A (2010) The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol 42(8):1316–1329. https://doi.org/10.1016/J.BIOCEL.2009.09.016
Jiang C, Li X, Zhao H, Liu H (2016) Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer 15(1):1–15. https://doi.org/10.1186/s12943-016-0545-z
Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J (2013) Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett 333(2):213–221. https://doi.org/10.1016/J.CANLET.2013.01.033
Takai D, Gonzales FA, Tsai YC, Thayer MJJP (2001) Large scale map** of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum Mol Genet 10(23):2619–2626
Pradella D, Naro C, Sette C, Ghigna C (2017) EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer 16(1):1–19. https://doi.org/10.1186/s12943-016-0579-2
Warzecha CC, Shen S, **ng Y, Carstens RP (2009) The epithelial splicing factors ESRP1 and ESRP2 positively and negatively regulate diverse types of alternative splicing events. RNA Biol 6(5):546–562. https://www.ncbi.nlm.nih.gov/pubmed/19829082
Warzecha CC, Jiang P, Amirikian K et al (2010) An ESRP-regulated splicing programme is abrogated during the epithelial–mesenchymal transition. EMBO J 29(19):3286 LP–3283300. http://emboj.embopress.org/content/29/19/3286.abstract
Sveen A, Kilpinen S, Ruusulehto A, Lothe RA, Skotheim RI (2015) Aberrant RNA splicing in cancer; expression changes and driver mutations of splicing factor genes. Oncogene 35:2413. https://doi.org/10.1038/onc.2015.318
Pattabiraman DR, Weinberg RA (2016) Targeting the epithelial-to-mesenchymal transition: the case for differentiation-based therapy. Cold Spring Harb Symp Quant Biol 81(1). https://doi.org/10.1101/sqb.2016.81.030957
Elaskalani O, Razak NBA, Falasca M, Metharom P (2017) Epithelial-mesenchymal transition as a therapeutic target for overcoming chemoresistance in pancreatic cancer. World J Gastrointest Oncol 9(1):37–41. https://doi.org/10.4251/wjgo.v9.i1.37
Singh M, Yelle N, Venugopal C, Singh SK (2018) EMT: mechanisms and therapeutic implications. Pharmacol Ther 182(August 2017):80–94. https://doi.org/10.1016/j.pharmthera.2017.08.009
Sarkar S, Goldgar S, Byler S, Rosenthal S, Heerboth S (2013) Demethylation and re-expression of epigenetically silenced tumor suppressor genes: sensitization of cancer cells by combination therapy. Epigenomics 5(1):87–94. https://doi.org/10.2217/epi.12.68
Heerboth S, Lapinska K, Snyder N, Leary M, Rollinson S, Sarkar S (2014) Use of epigenetic drugs in disease: an overview. Genet Epigenet 6:GEG.S12270. https://doi.org/10.4137/GEG.S12270
Sebestyén E, Singh B, Miñana B et al (2016) Large-scale analysis of genome and transcriptome alterations in multiple tumors unveils novel cancer-relevant splicing networks. Genome Res 26(6):732–744
Bonomi S, Gallo S, Catillo M, Pignataro D, Biamonti G, Ghigna C (2013) Oncogenic alternative splicing switches: role in cancer progression and prospects for therapy. Int J Cell Biol 2013:962038. https://doi.org/10.1155/2013/962038
Kole R, Krainer AR, Altman S (2012) RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov 11:125–140. https://doi.org/10.1038/nrd3625
Rigo F, Seth PP, Bennett CF (2014) Antisense oligonucleotide-based therapies for diseases caused by pre-mRNA processing defects. In: Yeo GW (ed) Systems biology of RNA binding proteins. Springer New York, New York, NY, pp 303–352. https://doi.org/10.1007/978-1-4939-1221-6_9
McClorey G, Wood MJ (2015) An overview of the clinical application of antisense oligonucleotides for RNA-targeting therapies. Curr Opin Pharmacol 24:52–58. https://doi.org/10.1016/J.COPH.2015.07.005
Sommers CL, Heckford SE, Skerker JM et al (1992) Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res 52(19):5190 LP–5195197. http://cancerres.aacrjournals.org/content/52/19/5190.abstract
Arumugam T, Ramachandran V, Fournier KF et al (2009) Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 69(14):5820 LP–5825828. http://cancerres.aacrjournals.org/content/69/14/5820.abstract
McConkey DJ, Choi W, Marquis L et al (2009) Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev 28(3):335–344. https://doi.org/10.1007/s10555-009-9194-7
Wahl GM, Spike BT (2017) Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. npj Breast Cancer 3(1). https://doi.org/10.1038/s41523-017-0012-z
Hölzel M, Bovier A, Tüting T (2013) Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer 13:365–376. https://doi.org/10.1038/nrc3498
Terry S, Savagner P, Ortiz-Cuaran S et al (2017) New insights into the role of EMT in tumor immune escape. Mol Oncol 11(7). https://doi.org/10.1002/1878-0261.12093
Housman G, Byler S, Heerboth S et al (2014) Drug resistance in cancer: an overview. Cancers 6(3). https://doi.org/10.3390/cancers6031769
Wan L, Pantel K, Kang Y (2013) Tumor metastasis: moving new biological insights into the clinic. Nat Med 19:1450–1464. https://doi.org/10.1038/nm.3391
Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018) EMT in cancer. Nat Rev Cancer 18:128–134. https://doi.org/10.1038/nrc.2017.118
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zilhão, R., Neves, H. (2019). Tumor Niche Disruption and Metastasis: The Role of Epithelial-Mesenchymal Transition (EMT). In: Fior, R., Zilhão, R. (eds) Molecular and Cell Biology of Cancer. Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-11812-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-11812-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11811-2
Online ISBN: 978-3-030-11812-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)