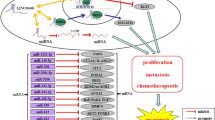
Abstract
Long non-coding RNAs (lncRNAs) play important roles in malignant neoplasia. Indeed, many hallmarks of cancer define that the malignant phenotype of tumor cells are controlled by lncRNAs. Despite a growing number of studies highlighting their importance in cancer, there has been no systematic review of metastasis-associated lncRNAs in various cancer types. Accordingly, we focus on the key metastasis-related lncRNAs and outline their expression status in cancer tissues by reviewing the previous stuides, in order to summarize the nowadays research achivements for lncRNAs related to cancer metastasis. Medline, EMBASE, as well as PubMed databases were applied to study lncRNAs which were tightly associated with tumor invasion and metastasis. Up to now, a substantial number of lncRNAs have been found to have important biological functions. In this review, according to their various features in cancer, lncRNAs were roughly divided into three categories: promoting tumor invasion and metastasis, negative regulation of tumor metastasis and with dual regulatory roles. The present studies may establish the foundation for both further research on the mechanisms of cancer progression and future lncRNA-based clinical applications.
Similar content being viewed by others
Background
The Encyclopedia of DNA Elements (ENCODE) project, the functional annotation of all regulatory regions of the human genome, has confirmed that 80 % of the genome is transcribed into RNA but less than 2 % is translated into proteins. Those RNA molecules that lack protein-coding capability are collectively referred to as non-coding RNA (NcRNA) [1–3]. These NcRNAs are divided into housekee** NcRNA and regulatory NcRNA [4], and, according to their molecular size, the latter can be subdivided into three major types: short NcRNA, mid-size NcRNA, and Long non-coding RNAs (lncRNAs) [5].
This review focuses on lncRNAs—those longer than 200 nucleotides (nt). Numerous studies have demonstrated that lncRNAs contribute to chromosome dosage-compensation, imprinting, epigenetic regulation, cell cycle control, nuclear and cytoplasmic trafficking, transcription, translation, splicing, and cell differentiation among other functions [6]. Studies also suggest that lncRNA aberrant expression is associated with numerous diseases including cancer. Currently, dozens of lncRNAs are implicated in the development and progression of cancer [7, 8]; hence, they may reveal novel mechanisms of transformation, tumor growth, and metastasis, as well as present new targets for cancer therapy.
Biological functions of lncRNAs
In the following sections, we will highlight the gene regulatory mechanisms and signaling pathways dependent on lncRNAs. The majority of lncRNAs described were involved in regulating the expression of protein-coding genes in cis (affecting neighboring genes) or in trans (affecting distant genes on different chromosomes) [6, 8]. LncRNAs control transcription, translation, and protein function at multiple levels. Various lncRNAs have been implicated in the regulation of individual genes as well as gene expression programs through epigenetic regulation or by altering the basal transcriptional machinery. Specifically, lncRNAs can (1) interfere with downstream gene expression by transcription at the upstream promoter region of protein-coding genes; (2) affect the expression of multiple downstream genes by inhibiting the activity of RNA polymerase II or via chromatin remodeling and histone modification; (3) disturb mRNA splicing patterns so as to produce different splice variants by complementary binding with pre-mRNAs; (4) modulate protein activity through direct binding; (5) function as scaffolds to form RNA-protein complexes; (6) regulate the subcellular localization of specific proteins; (7) serve as transcriptional precursors of small RNAs that can also regulate gene expression [9]. Due to this functional diversity, it is reasonable to speculate that over- or underexpression of lncRNAs can compromise a myriad of biological processes, thereby contributing to the pathogenesis of disease.
In fact, there is now beyond doubt that lncRNAs are of clinical importance, including for cancer biology and treatment. Many lncRNAs exhibit altered expression levels in cancer cells compared with healthy tissue of the same origin [10, 11]. Utilizing comparative mammalian genomics approach coupled with evolutionary analysis, Khachane and Harrison identified a small population of conserved long non-protein-coding RNAs (lncRNAs) in the evolution, and these lncRNAs could play an important role in cancer pathomechanisms [12]. Here we will highlight recent studies supporting the direct involvement of individual lncRNA molecules in the invasion and metastasis of various types of cancer.
LncRNAs in the invasion-metastasis cascade
Malignant tumors with infiltrative growth not only continuously grow and spread locally in situ, but also disseminate to other tissues through the lymphatic and circulatory systems as well as the body cavity via a process collectively referred to as tumor metastasis. Metastasis is one of the most significant biological characteristics of cancer and the leading cause of cancer-related death. Overexpression of NcRNAs are implicated in metastasis of human tumors, but most are shorter NcRNAs like microRNAs (miRNAs) [13]. However, recent studies have linked specific lncRNA gene mutations with cancer, raising the possibility of lncRNA-based cancer diagnostics and therapy. Dozens of lncRNAs are now widely believed to be involved in invasion and metastasis [14]. Therefore, this review will focus primarily on emerging mechanistic principles that underlie the nuclear functions of lncRNAs in order to provide oncologists with molecular insights that will help future research on lncRNAs in cancer management.
LncRNAs promote tumor invasion and metastasis
For the few lncRNAs that are characterized at the functional level, evidence is accumulating that they play pivotal roles in malignant diseases. Many features that define the malignant cell phenotype are controlled by lncRNAs, particularly the processes involved in metastasis. Below we describe several lncRNAs implicated in the etiopathology of malignant disorders, particularly those that distinguish highly aggressive tumors from indolent forms (summarized in Table 1).
HOTAIR
HOTAIR is a non-coding 2.2-kb RNA gene located downstream, in the antisense direction, of the gene encoding homeobox C12 (HOXC12). The HOTAIR lncRNA acts as a scaffold for histone modification complexes, allowing them to coordinately interact with the histone modifiers Polycomb Repressive Complex 2 (PRC2, a histone methyltransferase) and lysine-specific demethylase 1 (LSD1). In turn, HOTAIR guides these proteins to specific genomic regions and regulates gene expression through histone tail methylation. In cancer cells, HOTAIR partners with PRC2 to induce genome-wide gene silencing. Enhanced expression of HOTAIR has been found in both primary and metastatic lesions of multiple cancer types. In most cases, elevated HOTAIR expression was correlated with metastasis and poor prognosis [15]. As a cancer-associated lncRNA, HOTAIR is a prospective biomarker of metastasis and poor prognosis in a diverse group of cancers.
HOTAIR facilitates H3 lysine 27 methylation by PRC2 in a fibroblast cell model (producing H3K27). In situ, this pathway facilitated the invasion-metastasis cascade of breast cancer by repressing the expression of hox transcription factor protein D10 (homeobox D10 or HOXD10), progesterone receptor 1 (PRG1), cell adhesion molecules protocadherins (PCDHs), and tumor angiogenesis-related molecular ephrin receptors [16]. Recent studies have confirmed that HOTAIR expression levels differ substantially between primary and metastasized breast cancer, and so could be used as a prognostic marker [17].
HOTAIR is also frequently upregulated in oral squamous cell carcinoma and nasopharyngeal carcinoma, and overexpression is highly correlated with tumor metastasis, clinical stage, and poor prognosis. Multiple lines of evidence have established that HOTAIR was involved in a large range of biological processes and diseases, particularly in cancer development and metastasis [18, 19].
HOTAIR expression is higher in gastric cancer tissues than corresponding noncancerous tissues. Expression level is significantly related to larger tumor size, advanced pathological TNM stage, and distant metastasis, as well as shorter patient survival rate, particularly when associated with lymph node metastasis. HOTAIR knockdown efficiently inhibits cell proliferation and matrix invasiveness in gastric cancer cells in vitro [20–23].
In 78 cases of esophageal squamous cell carcinoma (ESCC) patients, almost all with high expression of HOTAIR in tumor tissues (75/78, 96.15 %), expression level correlated with tumor metastasis, TNM staging, and lower overall survival (OS) rate. The five-year survival rate of patients with positive HOTAIR expression was greatly reduced compared with patients with negative expression. In vitro, HOTAIR facilitates ESCC cell proliferation, colony formation, and migration [24], while silencing of HOTAIR in ESCC KYSE30 cells reduced cell invasion and migration but enhances apoptosis rate [25]. In nude mice ESCC models, HOTAIR promoted cell proliferation and tumor metastasis, while knockout reduced the metastasis of ESCC cells [26]. HOTAIR may promote ESCC cell metastasis by inhibiting the expression of Wnt inhibitory factor 1 (WIF-1), thereby activating the Wnt/β-catenin signaling pathway [27].
Higher HOTAIR expression is also observed in hepatocellular carcinoma (HCC) tissues compared to adjacent non-tumor tissues, and again expression level is associated with lymphatic metastasis. Depletion of HOTAIR in the HCC cell line Bel7402 reduces expression levels of matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth factor (VEGF), and consequently attenuates cell motility and metastasis [72].
UCA1
Urothelial carcinoma-associated 1 (UCA1), an oncofetal gene involved in embryonic development and carcinogenesis, often exhibits extraordinarily high expression in tumor tissues and cancer cells. In 94 cases of tongue squamous cell carcinoma (TSCC), expression of UCA1 was much higher than in adjacent normal samples. As expected, the ectopic expression of UCA1 induced a marked increase in lymph node metastasis [73].
Elevated levels of UCA1 are also found in ESCC tissues and the immortalized esophageal epithelial cell line NE1 compared to respective controls. UCA1 overexpression was often accompanied by tumor metastasis, suggesting that this lncRNA could be clinically useful as a diagnostic and prognostic indicator for ESCC patients [74].
In CRC tissues, high levels of UCA1 expression are correlated with larger tumor volume, greater invasion depth, less differentiated histology, and shorter survival. These results implicate UCA1 in the pathogenesis of CRC by enhancing the rates of cell growth, colonogenic survival, and the invasive and migratory potential of CRC cells [75]. Furthermore, under hypoxia, UCA1 levels are enhanced in bladder cancer cells compared to controls, and this high level of UCA1 expression is significantly correlated with greater tumor depth and apoptosis escape [76].
Expression of UCA1 is also higher in advanced stage (III/IV) compared to early stage (I/II) melanoma. UCA1 upregulation was correlated with poor differentiation, advanced lymph node classification, and metastasis of melanoma cells, while invasive and migratory capacities were remarkably diminished by UCA1 knockdown [57].
FOXCUT
FOXC1, a member of the Forkhead Box (FOX) family of transcription factors, is a key regulator of tumor occurrence and progression. The lncRNA-mRNA pair FOXCUT − FOXC1 may be a new functional form. Recent studies have demonstrated that high expression levels of FOXCUT and FOXC1 are strongly associated with poor prognosis in patients with base-like breast cancer (BLBC). Conversely, inhibition of FOXCUT impaired the invasion and migration capabilities of the breast cancer cell lines MDA-MB-231 and MDA-MB-468 [77].
A recent report indicated that FOXCUT was also more highly expressed in oral squamous cell carcinoma (OSCC) tissues compared to matching adjacent normal specimens and suggested a positive correlation with FOXC1. Transfection of Tca8113 and SCC-9 OSCC cell lines with a FOXCUT siRNA resulted in a substantially reduced number of colonies and lower migratory potential [78]. Furthermore, FOXCUT overexpression level was associated with tumor stage, metastasis, and post-operative survival of patients with ESCC. FOXCUT silencing in vitro using siRNA inhibited ESCC cell growth, colony formation, and dissemination compared to mock-transfected cells [79].
Other lncRNAs upregulated in tumor metastasis
Esophageal adenocarcinoma and esophageal squamous cell carcinoma
Expression levels of the lncRNAs AFAP1-AS1, HNF1A-AS1, and PEG10 are all markedly higher in malignant esophageal adenocarcinoma (ECA) tissues than in normal para-carcinoma samples, and expression correlates with cell motility, local infiltration depth, and vascular invasion as well as with tumor staging and differentiation level. On the contrary, knockdown of these lncRNAs lead to diminished cell viability and increased anoikis in EAC EC9706 and KYSE150 cells compared to cells transfected with empty vector [80–82].
Increased levels of the lncRNAs SPRY4-IT1 and TUG1 are observed in ESCC tissues and several ESCC cell lines. High SPRY4-IT1 expression level is positively correlated with FIGO stage, histological tumor grade, and lymph node metastasis. Suppression of the SPRY4-IT1 or TUG1 gene reduces invasive ability of ESCCs by blocking progression of the cell cycle [83, 84]. Khaitan et al. [85] found that local infiltration depth of neuroblastoma was correlated with dysregulated expression of SPRY4-IT1, leading to repressed proliferation and extensive apoptosis. This effect was mediated by multiple MAPK signaling molecules, including Raf1, B-Raf, MEK1/2, TESK1, MARKK, and MARK2. Thus, greater understanding of the underlying mechanisms of SPRY4-IT1 in the molecular etiology of ESCC could lead to major advances in lncRNA-directed diagnostics and define new therapeutic targets against this disease.
Gastric cancer and colorectal cancer
The lncRNA SDMGC (special for distant metastasis of GC) is an indicator of poor survival rate whilst its target gene TRIM16 is a positive prognostic factor in GC. Inhibition of SDMGC by RNAi decreased invasion of GC cells while suppression of TRIM16 has the opposite effect [86]. The lncRNA GAPLINC (gastric adenocarcinoma predictive long intergenic noncoding RNA) is also upregulated in GC patients, particularly with stage IV and distant metastasis. In addition, GAPLINC can function as a tumor promoter by upregulating CD44, a cell surface glycoprotein known to facilitate tumor metastasis [87].
Lately, it has been reported that lncRNAs regulated by transforming growth factor (TGF)-β (ATB) could initiate the invasion-metastasis cascade in CRC. Forced expression of ATB was closely related to bigger tumor volume, depth of tumor invasion, vascular and lymphatic invasion, as well as lymph node metastasis. In one study, patients with high ATB expression had strikingly shorter overall survivals than those with low expression [88]. Furthermore, higher ATB expression was detected in cancer patients with the transfer of blood-brone. Thus, ATB may be involved in CRC progression and could develop a promising indicator of worse prognosis [88].
Lung cancer
Lung cancer is the leading cause of cancer-related death and remains a major public health problem worldwide. Aside from HOTAIR and MALAT1, several other lncRNAs are correlated with lung cancer metastasis. LncRNA ZXF1 expression is markedly higher in lung adenocarcinoma tissues compared to adjacent non-cancerous lung tissues, and upregulated ZXF1 is correlated with the presence and extent of lymph node metastasis as well as tumor pathological stage. The three-year OS rate of patients with higher ZXF1 expression is reduced compared to patients with lower ZXF1, implying that high ZXF1 expression could be a marker for poor prognosis. In vitro, knockdown of ZXF1 by siRNA depressed the invasion and migration of A549 cells, whereas no remarkable effect was observed on cell growth [89].
LncRNA GHSROS (GHSR opposite strand) is transcribed from the antisense strand of the ghrelin receptor gene growth hormone secretagogue receptor (GHSR). Engineered overexpression of GHSROS stimulated cell migration in the A549 and NCI-H1299 NSCLC cell lines, but suppressed cell migration in the normal lung-derived bronchoepithelial cell line Beas-2B, indicating that GHSROS function may be dependent on the oncogenic context [90].
Cancer-associated region long non-coding RNA (CARLo-5) initially characterized in colon cancer was identified a fresh lncRNA. Subsequent studies have documented marked upregulation of CARLo-5 in NSCLC tissues compared to adjacent normal specimens. Moreover, patients with higher CARLo-5 expression levels have considerably worse prognosis than those with low expression. In vitro experiment, knockdown of CARLo-5 inhibited the proliferative activity, infiltration, and migration of NSCLC cell lines. Additionally, inhibition of CARLo-5 could reverse EMT in a NSCLC cell line. In summary, these results indicated that CARLo-5 may serve as a new prognostic marker and a potential molecular target in treatment of NSCLC [91].
LncRNA AF118081, a new oncogenic lncRNA, is the most highly overexpressed lncRNA in the transformed bronchial epithelial cell line 16HBE-T as well as in lung cancer specimens, while knockdown in 16HBE-T cells inhibited proliferation and invasion. In agreement with the results of in vitro assays, downregulation of AF118081 in a xenograft mouse model suppresses tumor growth [92].
Glioblastoma multiforme (GBM)
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain tumor. The lncRNA XIST (X-inactive specific transcript) is the master regulator of X inactivation in mammals. XIST expression is upregulated in glioma tissues and human glioblastoma stem cells (GSCs), while knockdown of XIST reduced cell proliferation, migration, and invasion, and induced apoptosis. In vivo knockdown of XIST restrained tumor growth and produced higher survival in nude mice. XIST could mediate its oncogenic effect at least in part by binding the tumor suppressor miR-152 [93].
Breast cancer
Commandeering of EMT regulatory pathways is a common mechanism whereby tumor cells depart from the primary site to invade surrounding tissue and establish distant metastases. Transforming growth factor beta (TGF-β) signaling is a major decoy of EMT capable of facilitating breast cancer metastasis, a process modulated by the lncRNA HIT (HOXA antisense transcript induced by TGF-β). HIT expression is dramatically increased in the highly metastatic 4 T1 cell line, and knockout of HIT in 4 T1 cells resulted in decreased cell migration and invasion. Moreover, higher HIT expression was associated with more invasive human breast carcinoma in patients [94].
Linc-ROR is a large intergenic non-coding RNA of about 2600 nt first described in induced pluripotent stem cells (iPSCs) and as a regulator of embryonic stem cell generation. Ectopic overexpression of linc-ROR enhanced breast cancer cell migration and invasion, whereas silencing repressed tumor growth and lung metastasis in vivo. Mechanistically, linc-ROR was associated with miRNPs (ribonucleoprotein complexes containing multiple miRNAs) and acted as a competitive endogenous RNA to miR-205, a member of the miRNA-200 family frequently suppressed in high-grade tumors. Specifically, linc-ROR prevented the degradation of miR-205 target genes, including the EMT inducer ZEB2. Thus, linc-ROR can operate as a chief regulator of EMT and advance the progression and metastasis of breast cancer through regulation of miRNAs [95].
The lncRNA Loc554202 gene at 9p21.3 does not contain an open reading frame, but rather is transcribed from four exons to create a spliced transcript of 2.2 kb. Loc554202 is expressed at markedly higher levels in breast cancer tissues than in normal controls and is associated with advanced pathologic stage and greater tumor size. Knockdown of Loc554202 depressed breast cancer cell proliferation, induced apoptosis, and inhibited migration/invasion in vitro as well as tumorigenesis in vivo [96].
LncRNA BCAR4 regulates a number of developmental and tumorigenic processes by activating a non-canonical Hedge-hog/GLI2 transcriptional program that promotes cell migration. Increased BCAR4 level was correlated with progressive mammary cancer, and targeting BCAR4 based on knockout of specific gene intensively suppressed breast cancer spread in an animal model [97].
Urologic neoplasms
The lncRNA RCCRT1 is upregulated in renal cell carcinoma (RCC) compared to adjacent noncancerous tissues, particularly in high-grade RCC tissues. Furthermore, siRNA-induced depletion of RCCRT1 expression diminished migration and invasion in ACHN and A498 RCC cell lines [98].
The lncRNA linc-UBC1a (up-regulated in bladder cancer 1a) was aberrantly expressed in a large cohort (103 cases) of bladder cancer and correlated with poor prognosis. Knockdown of linc-UBC1a decelerated the growth rate of bladder cancer cells, resulting in decreased tumor growth and metastasis [99]. LncRNA linc-UBC1a is identical to the previously reported cancer-upregulated drug resistant (CUDR) gene, which is known to play a pivotal role in both embryonic development and bladder cancer progression. Overexpression of UCA1a/CUDR elevated proliferation, migration, and invasion of the bladder cancer cell line UM-UC-2 in vitro [100].
SChLAP1 is abundantly expressed in approximately 25 % of prostate cancers and aids in the discrimination of aggressive tumors from indolent forms of the disease. SChLAP1 can coordinate cancer cell invasion in vitro and metastatic spread in vivo by impairing SNF5-mediated regulation of gene expression and genomic binding [101].
Linc00963 is upregulated in androgen-dependent LNCaP and androgen-independent C4-2 cell lines. Knockdown of linc00963 attenuated the expression of EGFR and the phosphorylation level of AKT even promoted apoptosis in C4-2 cells. These results suggested that Linc00963 prompted PCa transition from androgen-dependent to androgen-independent and metastasis via the EGFR signaling pathway [102].
The lncRNA PCGEM1 (Prostate cancer gene expression marker 1) has drawn increasing attention for its important role in PCa. PCGEM1 polymorphisms may contribute to PCa risk in Chinese men [103]. LncRNA PCAT18 is specifically expressed in PCa, and PCAT18 silencing significantly inhibited PCa cell proliferation, migration, and invasion, and triggered caspase 3/7 activation with no effect on non-neoplastic cells [104].
Female reproductive system tumors
LncRNA EBIC is another oncogenic lncRNA that regulates metastasis of cervical cancer by binding to the transcription repressor EZH2, thereby inhibiting E-cadherin expression [105]. LncRNA HOST2 (human ovarian cancer-specific transcript 2) is specifically overexpressed in human ovarian cancer. HOST2 inhibited miRNA let-7b function by reducing it bioavailability, leading to post-transcriptional suppression of oncogenes that regulated cell growth and motility [106]. The novel lincRNA ZNF300P1 is frequently hypermethylated in multiple ovarian cancer tissues and cell lines, and its expression can influence cell polarity, motility, and adhesion, while loss of expression may contribute to the metastatic potential of ovarian cancer cells [107].
EOC tissues expressing E2 receptor alpha (ERα) also express higher levels of lncRNA TC0101441 compared to ERα-negative tissues. Ectopic TC0101441 expression was correlated with lymph node metastasis, while knockdown impaired E2-induced EOC cell migration/invasion [108].
In aggregate, these findings implicate multiple lncRNAs as possible diagnostic and therapeutic targets for aggressive and metastatic cancers. In particular, the antitumor effects of targeted knockdown highlight the potential of lncRNA-based cancer therapies for patients at high risk for metastasis, an outcome currently lacking effective chemotherapeutic options.
LncRNAs involved in negative regulation of tumor metastasis
Compared to oncogenic lncRNAs, few lncRNAs have been confirmed as negative regulators of tumor invasion and metastasis. Such anti-tumor lncRNAs are discussed below along with their correlated expression in tumors (summarized in Table 2).
Lung cancer
In contrast to findings implicating BANCR in malignant melanoma, lung cancer, and CRC metastasis, BANCR levels are downregulated in NCI-H1688 and NCI-H446 lung carcinoma (LC) cell lines. When BANCR expression was strengthened by gain-of-function, tumor growth depressed and vice versa. In addition, BANCR was found to regulate LC proliferation and migration by inactivation of p38 MAPK and JNK [109].
Prostate cancer
The lncRNA DRAIC was identified by RNA sequencing (RNA-seq) and was found to be downregulated in the progression from androgen-dependent to castration-resistant PCa. Moreover, higher levels of DRAIC prevent PCa invasion/migration by modulating androgen receptor (AR) and FOXA1 expression, resulting in longer DFS [14]. Similar to DRAIC, lncRNA PCAT29 acts as a tumor suppressor via modulation of AR [110]. Thus, these two lncRNAs may act as androgen-regulated tumor suppressors in PCa.
Neurospongioma
The lncRNA TSLC1-AS1 is the antisense transcript of tumor suppressor TSLC1. Its expression level is notably lower in glioma tissues. In glioblastoma U87 cells, the overexpression of TSLC1-AS1 upregulated TSLC1 and inhibited cell proliferation, migration, and invasion, while TSLC1-AS1 silencing has the opposite effects in human neuroglioma SNB-19 cells. These results suggest that TSLC1-AS1 may serve as a potential biomarker and therapeutic target for glioma by interfering with TSLC1 expression [111]. The lncRNA ADAMTS9-AS2 is the antisense transcript of tumor suppressor ADAMTS9. ADAMTS9-AS2 is also a glioma suppressor as expression is correlated with lower tumor grade and better prognosis. ADAMTS9-AS2 can modify malignant glioma behavior through DNA methyltransferase-1 [112].
LncRNA CASC2 (cancer susceptibility candidate 2) was originally described as a tumor suppressor gene in endometrial and colorectal cancers. Low expression levels have also been found in glioma tissues as well as in U251 and U87 glioma cell lines. Overexpression of CASC2 may hinder glioma progression by negative regulation of miR-21 [113].
Head and neck squamous cell carcinoma
Application of next-generation RNA-seq revealed 2808 differentially expressed lncRNAs between 40 head and neck squamous cell carcinoma and paired normal tissues. The expression levels of lncRNA LCE5A-1 and KCTD6-3 were markedly lower in head and neck neoplasm tissues and closely associated with the prognosis of patients. In head neck squamous cell carcinoma cells, overexpression of these two lncRNAs diminished cell growth and metastasis by regulating EMT-related gene expression and inhibiting tumor stem cell functions [114].
Renal cell carcinoma
The lncRNA growth-arrest-specific 5 (Gas5) sensitizes the cell to apoptosis by regulating the activity of glucocorticoids in response to nutrient starvation. Gas5 has also been linked to RCC as transcript levels are significantly reduced compared to unaffected normal renal tissues. Overexpression of GAS5 in A498 cells induced apoptosis and cell motility by acting as a transcription factor decoy for steroid hormone receptors [115]. LncRNA CADM1-AS1 expression is downregulated in tumor tissues of 64 patients with ccRCC, the most common RCC subtype, compared to adjacent non-tumor tissue. Furthermore, CADM1-AS1 expression was positively correlated with CADM1 mRNA expression in ccRCC specimens as well as 786-O and ACHN renal carcinoma cells. Thus, CADM1-AS1 was likely a ccRCC tumor suppressor that regulates cell proliferation, apoptosis, and migration via CADM1 [116].
Hepatocellular carcinoma and gastric cancer
The lncRNA PTENP1 modulates HCC cell behavior and gene networks by miRNA regulation, including the oncomirs miR-17, miR-19b, and miR-20a, as well as through modulation of the PI3K/AKT pathway. Overexpression of PTENP1 mitigated tumor growth, suppressed intratumoral cell proliferation, elicited apoptosis and autophagy, and inhibited angiogenesis in an animal model [117].
The lncRNA FENDRR controls the expression of target genes epigenetically by binding to PRC2. Low expression of FENDRR occured in GC cell lines and tissues and was associated with poor prognosis. FENDER overexpression suppressed invasion and migration in GC cells in vitro by attenuating FN1 and MMP2/MMP9 gene expression [118].
Pancreatic cancer
LncRNA ENST00000480739 expression is dramatically decreased in pancreatic ductal adenocarcinoma (PDAC), which contributed to tumor metastasis and progression by modulating HIF-1α. ENST00000480739 level was negatively related to tumor node metastasis stage and lymph node metastasis, indicating that it could be an independent prognostic factor of survival time in PDAC patients following surgery [119]. Further, enhanced ENST00000480739 expression in vitro inhibited PDAC cell invasion [119].
The lncRNA maternally expressed gene 3 (Meg3) was characterized as a tumor suppressor in pancreatic neuroendocrine tumor (PNET) cells. Overexpression of Meg3 in the insulin-secreting mouse PNET cell line MIN6 prevented proliferation and delayed cell cycle progression. Microarray studies have found that upregulated expression of Meg3 in MIN6 cells can attenuate the level of the proto-oncogene c-Met and reduce cell migration and invasion [120].
Dual function of lncRNAs
Only a few of the lncRNAs characterized to date have shown dual tumor promotion and tumor suppressor functions. As mentioned earlier, the 693-bp lncRNA BANCR appears to both enhance and suppress metastatic potential. BANCR expression was elevated in GC tissues compared to matched non-cancerous tissue but downregulated in 113 NSCLC tumor tissues compared to match normal samples. Moreover, the aberrant expression of BANCR was positively associated with clinical stage, tumor depth, lymph node metastasis, and distant metastasis, and was an independent prognostic factor for GC/NSCLC in survival analysis [121, 122]. H19 has also been found to have both oncogenic and suppressive properties in different tumors [123]. While several studies reported that H19 functioned as an oncogenic lncRNA promoting tumor metastasis in GC, ovarian cancer, endometrial cancer, and bladder cancer, other studies found that it inhibits tumor metastasis via miR-675. Both H19 and H19-induced miR-675 expression level were significantly lower in metastatic PCa than less aggressive PCa. The H19/miR-675 axis may have diagnostic and therapeutic potential for advanced PCa cases [124]. Conversely, a miR-675 inhibitor or H19 siRNA dramatically increases HCC cell migration and invasion via the AKT/GSK-3β/Cdc25A signaling pathway [123]. The dual functionality of certain lncRNAs may stem from differential co-expression of other targets or tumor regulators in specific cell types. The ‘off-target’ effects of lncRNAs via indirect regulation or negative feedback look of gene-expression would be one potential reason.
Conclusions and perspectives
At present, a large number of deregulated lncRNAs are involved in proliferation, apoptosis and cell-cycle control in the carcinogenesis, there is a growing list of lncRNAs that contribute to the specific process of metastasis. Some well-studied lncRNAs were demonstrated to be differentially expressed in metastatic tumor foci. In less than a decade of research on lncRNAs, as well as their prominent role in the regulation of tumor progression, have revealed the huge potential of the long non-coding transcriptome for therapeutic intervention, both as targets (when they are upregulated) and as drugs (when they are downregulated or lost). Yet in spite of lncRNAs becoming a research hotspot gradually, conceptual and technical limitations have restricted our deeper understanding on their contributions to human malignant tumors. Initially, the key factors for future development are to improve computational approaches and databases to distinguish potential functional and pathological links between lncRNAs and tumor occurrence and metastasis. In addition, transporting ncRNAs only to potentially cancerous cells is surely another main conundrum. It may be more complicate matters considering the size of lncRNAs when compared with miRNAs and siRNAs, bringing greater challenges such as delivery and stability to the target. However, nanoparticles and liposomes may be reconstructed in order to achieve tumor-targeting drug delivery, in an attempt to keep harmful side-effects remained manageable. Lastly, few animal models have been well developed to appraise the roles of lncRNAs in vivo and even as we know, there is still no lncRNA that enters clinical trials for the moment. The retardation of animal assays and human clinical trials could greatly impede the development and utilization of lncRNA-based new drugs and this will be another focus and difficulty of our research in the future.
Therefore, an absolute requirement is a more profound awareness of lncRNA functions and mechanisms, both in physiological and pathological circumstances. It is now necessary to expand and reconstruct lncRNA-enriched disease networks to develop new therapeutic strategies, allowing to increase the specificity and to decrease the toxicity especially in cancer therapy, so that lncRNA-based diagnostics and targeted therapeutics in cancer and metastasis can safely and successfully enter into regular clinical practice.
Abbreviations
- AKAP-9:
-
PRKA kinase anchor protein 9
- AR:
-
Androgen receptor
- BANCR:
-
BRAF-activated non-coding RNA
- BLBC:
-
Base-like breast cancer
- CASC2:
-
Cancer susceptibility candidate 2
- CCAT1:
-
Colon cancer associated transcript 1
- ccRCC:
-
Clear cell renal cell carcinoma
- CRC:
-
Colorectal cancer
- CUDR:
-
Cancer-upregulated drug resistant
- DFS:
-
Disease-free survival
- ECA:
-
Esophageal adenocarcinoma
- EMT:
-
Epithelial-to-mesenchymal transition
- EOC:
-
Epithelial ovarian cancer
- ERα:
-
E2 receptor alpha
- ESCC:
-
Esophageal squamous cell carcinoma
- EZH2:
-
Enhancer of zeste homolog 2
- FOX:
-
Forkhead box
- Gas5:
-
Growth-arrest-specific 5
- GBM:
-
Glioblastoma multiforme
- GC:
-
Gastric cancer
- GHSR:
-
Growth hormone secretagogue receptor
- GSCs:
-
Glioblastoma stem cells
- HCC:
-
Hepatocellular carcinoma
- HOST2:
-
Human ovarian cancer-specific transcript 2
- HOXC12:
-
Homeobox C12
- HOXD10:
-
Hox transcription factor protein D10
- LNAs:
-
Locked nucleic acids
- lncRNA:
-
Long non-coding RNA
- LSD1:
-
Lysine-specific demethylase 1
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- Meg3:
-
Maternally expressed gene 3
- miRNA:
-
microRNA
- MMP-9:
-
Matrix metalloproteinase-9
- MMPs:
-
Matrix metalloproteinases
- NcRNA:
-
Non-coding RNA
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- OSCC:
-
Oral squamous cell carcinoma
- PCa:
-
Prostate cancer
- PCDHs:
-
Cell adhesion molecules protocadherins
- PCGEM1:
-
Prostate cancer gene expression marker 1
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PNET:
-
Pancreatic neuroendocrine tumor
- PRC2:
-
Polycomb repressive complex 2
- PRG1:
-
progesterone receptor 1
- RACE:
-
Rapid amplification of cDNA ends
- RBM38:
-
RNA binding motif 38
- RCC:
-
Renal cell carcinoma
- RDA:
-
RNA disruption assay
- RNAi:
-
RNA interference
- TGF-β:
-
Transforming growth factor beta
- TSCC:
-
Tongue squamous cell carcinoma
- UCA1:
-
Urothelial carcinoma-associated 1
- VEGF:
-
Vascular endothelial growth factor
- WIF-1:
-
Wnt inhibitory factor 1
References
Ponting CP, Belgard TG. Transcribed dark matter: meaning or myth? Hum Mol Genet. 2010;19(R2):R162–8.
Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA biology. 2012;9(6):703–19.
Nie L, Wu HJ, Hsu JM, Chang SS, Labaff AM, Li CW, et al. Long non-coding RNAs: versatile master regulators of gene expression and crucial players in cancer. Am J Transl Res. 2012;4(2):127–50.
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–9.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74.
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–7.
Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10(1):38.
Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21(6):354–61.
Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–504.
Gibb EA, Vucic EA, Enfield KSS, Stewart GL, Lonergan KM, Kennett JY, et al. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6(10):e25915.
Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, et al. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis. 2013;34(6):1343–51.
Khachane AN, Harrison PM. Mining mammalian transcript data for functional long non-coding RNAs. PLoS One. 2010;5(4):e10316.
Bullock MD, Sayan AE, Packham GK, Mirnezami AH. MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biology of the cell/under the auspices of the European Cell Biology Organization. 2012;104(1):3–12.
Sakurai K, Reon BJ, Anaya J, Dutta A. The lncRNA DRAIC/PCAT29 locus constitutes a tumor-suppressive nexus. Mol Cancer Res. 2015;13(5):828–38.
Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–25.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA biology. 2010;7(5):582–5.
Wu Y, Zhang L, Zhang L, Wang Y, Li H, Ren X, et al. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol. 2015;46(6):2586–94.
Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104(4):458–64.
Lee NK, Lee JH, Park CH, Yu D, Lee YC, Cheong JH, et al. Long non-coding RNA HOTAIR promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem Biophys Res Commun. 2014;451(2):171–8.
Wang J, Song YX, Wang ZN. Non-coding RNAs in gastric cancer. Gene. 2015;560(1):1–8.
Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35(12):2731–9.
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92.
Lv XB, Lian GY, Wang HR, Song E, Yao H, Wang MH. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS One. 2013;8(5):e63516.
Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu ZL, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol Carcinog. 2013;52(11):908–15.
Li X, Wu Z, Mei Q, Li X, Guo M, Fu X, et al. Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer. 2013;109(8):2266–78.
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY, Xue WQ, et al. HOTAIR, a prognostic factor in esophageal squamous cell carcinoma, inhibits WIF-1 expression and activates Wnt pathway. Cancer Sci. 2013;104(12):1675–82.
Geng YJ, **e SL, Li Q, Ma J, Wang GY. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;39(6):2119–28.
Ding C, Cheng S, Yang Z, Lv Z, **ao H, Du C, et al. Long non-coding RNA HOTAIR promotes cell migration and invasion via down-regulation of RNA binding motif protein 38 in hepatocellular carcinoma cells. Int J Mol Sci. 2014;15(3):4060–76.
Zhao W, An Y, Liang Y, **e XW. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci. 2014;18(13):1930–6.
Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K, Tanaka N, et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436(2):319–24.
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464.
Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim SW, et al. Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int J Oncol. 2015;46(2):521–30.
**g L, Yuan W, Ruofan D, **** Y, Haifeng Q. HOTAIR enhanced aggressive biological behaviors and induced radio-resistance via inhibiting p21 in cervical cancer. Tumour Biol. 2015;36(5):3611–9.
Huang J, Ke P, Guo L, Wang W, Tan H, Liang Y, et al. Lentivirus-mediated RNA interference targeting the long noncoding RNA HOTAIR inhibits proliferation and invasion of endometrial carcinoma cells in vitro and in vivo. Int J Gynecol Cancer. 2014;24(4):635–42.
Qiu JJ, Lin YY, Ye LC, Ding JX, Feng WW, ** HY, et al. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol. 2014;134(1):121–8.
Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8(8):e70372.
Wu ZH, Wang XL, Tang HM, Jiang T, Chen J, Lu S, et al. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32(1):395–402.
Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression [J]. Cancer Res. 2011;71(1):3–7.
Vassallo I, Zinn P, Lai M, Rajakannu P, Hamou MF, Hegi ME. WIF1 re-expression in glioblastoma inhibits migration through attenuation of non-canonical WNT signaling by downregulating the lncRNA MALAT1. Oncogene. 2016;35:12-21.
Tee AE, Ling D, Nelson C, Atmadibrata B, Dinger ME, Xu N, et al. The histone demethylase JMJD1A induces cell migration and invasion by up-regulating the expression of the long noncoding RNA MALAT1. Oncotarget. 2014;5(7):1793–804.
Zhao Z, Chen C, Liu Y, Wu C. 17β-Estradiol treatment inhibits breast cell proliferation, migration and invasion by decreasing MALAT-1 RNA level. Biochem Biophys Res Commun. 2014;445(2):388–93.
Schmidt LH, Spieker T, Koschmieder S, Schaffers S, Humberg J, Jungen D, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6(12):1984–92.
Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73(3):1180–9.
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y, et al. Up-regulation of long noncoding RNA MALAT1 contributes to proliferation and metastasis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2015;34:7.
Wang X, Li M, Wang Z, Han S, Tang X, Ge Y, et al. Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J Biol Chem. 2015;290(7):3925–35.
Jiao F, Hu H, Yuan C, Wang L, Jiang W, ** Z, et al. Elevated expression level of long noncoding RNA MALAT-1 facilitates cell growth, migration and invasion in pancreatic cancer. Oncol Rep. 2014;32(6):2485–92.
Zhang HM, Yang FQ, Chen SJ, Che J, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36(4):2947–55.
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, et al. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20(6):1531–41.
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8(9):2289–94.
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190(6):2278–87.
Xu C, Yang M, Tian J, Wang X, Li Z. MALAT-1: a long non-coding RNA and its important 3′ end functional motif in colorectal cancer metastasis. Int J Oncol. 2011;39(1):169–75.
Yang MH, Hu ZY, Xu C, **e LY, Wang XY, Chen SY, et al. MALAT1 promotes colorectal cancer cell proliferation/migration/invasion via PRKA kinase anchor protein 9. Biochim Biophys Acta. 2015;1852(1):166–74.
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111(4):736–48.
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS One. 2013;8(11):e78700.
Fang D, Yang H, Lin J, Teng Y, Jiang Y, Chen J, et al. 17beta-estradiol regulates cell proliferation, colony formation, migration, invasion and promotes apoptosis by upregulating miR-9 and thus degrades MALAT-1 in osteosarcoma cell MG-63 in an estrogen receptor-independent manner. Biochem Biophys Res Commun. 2015;457(4):500–6.
Tian Y, Zhang X, Hao Y, Fang Z, He Y. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res. 2014;24(4):335–41.
Chen F, Tian Y, Pang EJ, Wang Y, Li L. MALAT2-activated long noncoding RNA indicates a biomarker of poor prognosis in gastric cancer. Cancer Gene Ther. 2015. doi:10.1038/cgt.2015.6.
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, et al. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279(17):3159–65.
Zhang EB, Han L, Yin DD, Kong R, De W, Chen J. c-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014;31(5):914.
Li H, Yu B, Li J, Su L, Yan M, Zhu Z, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5(8):2318–29.
Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z, et al. H19 promotes pancreatic cancer metastasis by derepressing let-7's suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014;35(9):9163–9.
Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S, et al. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene. 2015;34(23):3076–84.
Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333(2):213–21.
Adriaenssens E, Lottin S, Berteaux N, Hornez L, Fauquette W, Fafeur V, et al. Cross-talk between mesenchyme and epithelium increases H19 gene expression during scattering and morphogenesis of epithelial cells. Exp Cell Res. 2002;275(2):215–29.
Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, et al. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome research. 2012;22(6):1006–14.
Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. 2015;36:7205–11.
Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang D, et al. BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. Oncol Lett. 2014;8(2):869–75.
Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34:18.
Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, et al. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139(3):437–45.
Redis RS, Sieuwerts AM, Look MP, Tudoran O, Ivan C, Spizzo R, et al. CCAT2, a novel long non-coding RNA in breast cancer: expression study and clinical correlations. Oncotarget. 2013;4(10):1748–62.
Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA map** to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome research. 2013;23(9):1446–61.
Fang Z, Wu L, Wang L, Yang Y, Meng Y, Yang H. Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: a possible correlation with cancer metastasis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):89–95.
Li JY, Ma X, Zhang CB. Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(11):7938–44.
Han Y, Yang YN, Yuan HH, Zhang TT, Sui H, Wei XL, et al. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46(5):396–401.
Xue M, Li X, Li Z, Chen W. Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1alpha-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol. 2014;35(7):6901–12.
Liu J, Shen L, Yao J, Li Y, Wang Y, Chen H, et al. Forkhead box C1 promoter upstream transcript, a novel long non-coding RNA, regulates proliferation and migration in basal-like breast cancer. Mol Med Rep. 2015;11(4):3155–9.
Kong XP, Yao J, Luo W, Feng FK, Ma JT, Ren YP, et al. The expression and functional role of a FOXC1 related mRNA-lncRNA pair in oral squamous cell carcinoma. Mol Cell Biochem. 2014;394(1–2):177–86.
Pan F, Yao J, Chen Y, Zhou C, Geng P, Mao H, et al. A novel long non-coding RNA FOXCUT and mRNA FOXC1 pair promote progression and predict poor prognosis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(6):2838–49.
Wu W, Bhagat TD, Yang X, Song JH, Cheng Y, Agarwal R, et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 2013;144(5):956–66. e4.
Yang X, Song JH, Cheng Y, Wu W, Bhagat T, Yu Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63(6):881–90.
Zang W, Wang T, Huang J, Li M, Wang Y, Du Y, et al. Long noncoding RNA PEG10 regulates proliferation and invasion of esophageal cancer cells. Cancer Gene Ther. 2015;22(3):138–44.
**e HW, Wu QQ, Zhu B, Chen FJ, Ji L, Li SQ, et al. Long noncoding RNA SPRY4-IT1 is upregulated in esophageal squamous cell carcinoma and associated with poor prognosis. Tumour Biol. 2014;35(8):7743–54.
Xu Y, Wang J, Qiu M, Xu L, Li M, Jiang F, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumour Biol. 2015;36(3):1643–51.
Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS, et al. The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res. 2011;71(11):3852–62.
Yan Y, Shen Z, Gao Z, Cao J, Yang Y, Wang B, et al. Long noncoding ribonucleic acid specific for distant metastasis of gastric cancer is associated with TRIM16expression and facilitates tumor cell invasion in vitro. J Gastroenterol Hepatol. 2015;30(9):1367-75.
Hu Y, Wang J, Qian J, Kong X, Tang J, Wang Y, et al. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 2014;74(23):6890–902.
Iguchi T, Uchi R, Nambara S, Saito T, Komatsu H, Hirata H, et al. A Long Noncoding RNA, lncRNA-ATB, Is Involved in the Progression and Prognosis of Colorectal Cancer. Anticancer Res. 2015;35(3):1385–8.
Zhang L, Zhou XF, Pan GF, Zhao JP. Enhanced expression of long non-coding RNA ZXF1 promoted the invasion and metastasis in lung adenocarcinoma. Biomed Pharmacother. 2014;68(4):401–7.
Whiteside EJ, Seim I, Pauli JP, O’Keeffe AJ, Thomas PB, Carter SL, et al. Identification of a long non-coding RNA gene, growth hormone secretagogue receptor opposite strand, which stimulates cell migration in non-small cell lung cancer cell lines. Int J Oncol. 2013;43(2):566–74.
Luo J, Tang L, Zhang J, Ni J, Zhang HP, Zhang L, et al. Long non-coding RNA CARLo-5 is a negative prognostic factor and exhibits tumor pro-oncogenic activity in non-small cell lung cancer. Tumour Biol. 2014;35(11):11541–9.
Yang Q, Zhang S, Liu H, Wu J, Xu E, Peng B, et al. Oncogenic role of long noncoding RNA AF118081 in anti-benzo [a] pyrene-trans-7, 8-dihydrodiol-9, 10-epoxide-transformed 16HBE cells. Toxicol Lett. 2014;229(3):430–9.
Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J, et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015;359(1):75–86.
Richards EJ, Zhang G, Li ZP, Permuth-Wey J, Challa S, Li Y, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) beta: LncRNA-hit-mediated TGFbeta-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015;290(11):6857–67.
Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, et al. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287.
Shi Y, Lu J, Zhou J, Tan X, He Y, Ding J, et al. Long non-coding RNA Loc554202 regulates proliferation and migration in breast cancer cells. Biochem Biophys Res Commun. 2014;446(2):448–53.
**ng Z, Lin A, Li C, Liang K, Wang S, Liu Y, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell. 2014;159(5):1110–25.
Song S, Wu Z, Wang C, Liu B, Ye X, Chen J, et al. RCCRT1 is correlated with prognosis and promotes cell migration and invasion in renal cell carcinoma. Urology. 2014;84(3):730. e1-7.
He W, Cai Q, Sun F, Zhong G, Wang P, Liu H, et al. linc-UBC1 physically associates with polycomb repressive complex 2 (PRC2) and acts as a negative prognostic factor for lymph node metastasis and survival in bladder cancer. Biochim Biophys Acta. 2013;1832(10):1528–37.
Wang Y, Chen W, Yang C, Wu W, Wu S, Qin X, et al. Long non-coding RNA UCA1a (CUDR) promotes proliferation and tumorigenesis of bladder cancer. Int J Oncol. 2012;41(1):276–84.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, et al. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16(12):1121–7.
Wang L, Han S, ** G, Zhou X, Li M, Ying X, et al. Linc00963: a novel, long non-coding RNA involved in the transition of prostate cancer from androgen-dependence to androgen-independence. Int J Oncol. 2014;44(6):2041–9.
He JH, Zhang JZ, Han ZP, Wang L, Lv YB, Li YG. Reciprocal regulation of PCGEM1 and miR-145 promote proliferation of LNCaP prostate cancer cells. J Exp Clin Cancer Res. 2014;33:72.
Crea F, Watahiki A, Quagliata L, Xue H, Pikor L, Parolia A, et al. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget. 2014;5(3):764–74.
Sun NX, Ye C, Zhao Q, Zhang Q, Xu C, Wang SB, et al. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One. 2014;9(7):e100340.
Gao Y, Meng H, Liu S, Hu J, Zhang Y, Jiao T, et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum Mol Genet. 2015;24(3):841–52.
Gloss B, Moran-Jones K, Lin V, Gonzalez M, Scurry J, Hacker NF, et al. ZNF300P1 encodes a lincRNA that regulates cell polarity and is epigenetically silenced in type II epithelial ovarian cancer. Mol Cancer. 2014;13:3.
Qiu J, Ye L, Ding J, Feng W, Zhang Y, Lv T, et al. Effects of oestrogen on long noncoding RNA expression in oestrogen receptor alpha-positive ovarian cancer cells. J Steroid Biochem Mol Biol. 2014;141:60–70.
Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang L, et al. Long non-coding RNA BANCR promotes proliferation and migration of lung carcinoma via MAPK pathways. Biomed Pharmacother. 2015;69:90–5.
Malik R, Patel L, Prensner JR, Shi Y, Iyer MK, Subramaniyan S, et al. The lncRNA PCAT29 inhibits oncogenic phenotypes in prostate cancer. Mol Cancer Res. 2014;12(8):1081–7.
Qin X, Yao J, Geng P, Fu X, Xue J, Zhang Z. LncRNA TSLC1-AS1 is a novel tumor suppressor in glioma. Int J Clin Exp Pathol. 2014;7(6):3065–72.
Yao J, Zhou B, Zhang J, Geng P, Liu K, Zhu Y, et al. A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated by DNMT1 and inhibits migration of glioma cells. Tumour Biol. 2014;35(8):7935–44.
Wang P, Liu YH, Yao YL, Li Z, Li ZQ, Ma J, et al. Long non-coding RNA CASC2 suppresses malignancy in human gliomas by miR-21. Cell Signal. 2015;27(2):275–82.
Zou AE, Ku J, Honda TK, Yu V, Kuo SZ, Zheng H, et al. Transcriptome sequencing uncovers novel long noncoding and small nucleolar RNAs dysregulated in head and neck squamous cell carcinoma. RNA. 2015;21(6):1122–34.
Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14(2):1077–82.
Yao J, Chen Y, Wang Y, Liu S, Yuan X, Pan F, et al. Decreased expression of a novel lncRNA CADM1-AS1 is associated with poor prognosis in patients with clear cell renal cell carcinomas. Int J Clin Exp Pathol. 2014;7(6):2758–67.
Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC, Hwang SM, et al. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71–81.
Xu TP, Huang MD, **a R, Liu XX, Sun M, Yin L, et al. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol. 2014;7:63.
Sun YW, Chen YF, Li J, Huo YM, Liu DJ, Hua R, et al. A novel long non-coding RNA ENST00000480739 suppresses tumour cell invasion by regulating OS-9 and HIF-1alpha in pancreatic ductal adenocarcinoma. Br J Cancer. 2014;111(11):2131–41.
Modali SD, Parekh VI, Kebebew E, Agarwal SK. Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol. 2015;29(2):224–37.
Li L, Zhang L, Zhang Y, Zhou F. Increased expression of LncRNA BANCR is associated with clinical progression and poor prognosis in gastric cancer. Biomed Pharmacother. 2015;72:109–12.
Sun M, Liu XH, Wang KM, Nie FQ, Kong R, Yang JS, et al. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol Cancer. 2014;13:68.
Lv J, Ma L, Chen XL, Huang XH, Wang Q. Downregulation of LncRNAH19 and MiR-675 promotes migration and invasion of human hepatocellular carcinoma cells through AKT/GSK-3beta/Cdc25A signaling pathway. J Huazhong Univ Sci Technolog Med Sci. 2014;34(3):363–9.
Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng R, et al. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281(16):3766–75.
Acknowledgements
None.
Funding
This work was supported by grants from the National Nature Science Foundation of China (No. 81560415), Science and technology support project of **njiang Uygur Autonomous Region (No. 2016E02071).
Availability of data and material
Medline, EMBASE, as well as PubMed databases were applied.
Authors’ contributions
CYJ, HBL and XL conceived, designed paper writing, carried out the references collection and wrote the paper; HZ and HBL guided the analysis for references; CYJ and HZ prepared all tables; HBL, CYJ and XL supervised and directed the process of the review writing. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors consented for publication.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jiang, C., Li, X., Zhao, H. et al. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer 15, 62 (2016). https://doi.org/10.1186/s12943-016-0545-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-016-0545-z