Abstract
Brown adipose tissue is specialized to burn lipids for thermogenesis and energy expenditure. Second-generation antipsychotics (SGA) are the most commonly used drugs for schizophrenia with several advantages over first-line drugs, however, it can cause clinically-significant weight gain. To reveal the involvement of brown adipocytes in SGA-induced weight gain, we compared the effect of clozapine, quetiapine, and ziprasidone, SGA with different propensities to induce weight gain, on the differentiation and the expression of brown fat-specific markers, lipogenic genes and adipokines in a mouse brown preadipocyte cell line. On Oil Red-O staining, the differentiation was inhibited almost completely by clozapine (40 µM) and partially by quetiapine (30 µM). Clozapine significantly down-regulated the brown adipogenesis markers PRDM16, C/EBPβ, PPARγ2, UCP-1, PGC-1α, and Cidea in dose- and time-dependent manners, whereas quetiapine suppressed PRDM16, PPARγ2, and UCP-1 much weakly than clozapine. Clozapine also significantly inhibited the mRNA expressions of lipogenic genes ACC, SCD1, GLUT4, aP2, and CD36 as well as adipokines such as resistin, leptin, and adiponectin. In contrast, quetiapine suppressed only resistin and leptin but not those of lipogenic genes and adiponectin. Ziprasidone (10 µM) did not alter the differentiation as well as the gene expression patterns. Our results suggest for the first time that the inhibition of brown adipogenesis may be a possible mechanism to explain weight gain induced by clozapine and quetiapine.
Similar content being viewed by others
Introduction
Second-generation antipsychotics (SGA) are the most common drugs used for treatment of schizophrenia and have a reduced risk of extrapyramidal symptoms compared to first-generation antipsychotics. However, most SGA are linked to weight gain, impaired glucose tolerance and lipid abnormalities, resulting in the development of various metabolic disorders. A high prevalence of metabolic syndrome (McEvoy et al., 2005; Birkenaes et al., 2007) and an increased mortality rate (Laursen et al., 2007) were observed in schizophrenic patients. The risk of weight gain is variable among SGA. Olanzapine and clozapine are associated with the highest risk of weight gain, followed by chloropromzaine, resperidone, and quetiapine (Lett et al., 2012). Ziprasidone belongs to the group with the lowest risk of weight gain, and it has even shown to reduce weight gain by increasing resting energy expenditure without decreasing food intake (Park et al., 2012). The rank order of SGA for inducing weight gain is consistent with that of their metabolic side effects (Newcomer, 2005, 2007), supporting the idea that drug's risk for adverse metabolic changes are strongly associated with its potency for increasing adiposity. Although differential binding affinity of individual SGA to histamine, serotonin, α-adrenergic receptors (Kroeze et al., 2003; Matsui-Sakata et al., 2005; Deng et al., 2010) or PKC-β activation (Pavan et al., 2010) has been suggested as possible mechanisms, the biochemical and pharmacological bases of SGA-induced weight gain are mostly unclear.
Brown adipose tissue (BAT) serves a specialized function in adaptive thermogenesis and plays an opposite role to that of white adipose tissue by burning metabolic substrates instead of storing energy in the form of triglycerides. Therefore, BAT has long been accepted as an important metabolic organ in small mammals such as mice or rats, but was previously considered to have little physiological relevance in humans beyond early childhood. However, several lines of evidence using 18F-fluorodeoxyglucose positron emission tomography clearly confirmed the existence of active BAT in humans (Nedergaard et al., 2007; Lichtenbelt et al., 2009). Moreover, recent studies also suggested a possible role of BAT in human energy metabolism. BAT activation is either stimulated by cold, insulin, and catecholamines (Orava et al., 2011; Wang et al., 2011) or down-regulated by central obesity and bariatric surgery (Wang et al., 2011; Vijgen et al., 2012). Promoting brown adipocyte differentiation leads to an increase in energy expenditure and a reduction in weight gain (Tseng et al., 2008), whereas reduced brown adipocyte differentiation is associated with obesity and insulin resistance in humans (Yang et al., 2003). To the best of our knowledge, only two reports have suggested a possible involvement of BAT in psychotropic-induced obesity. Lithium, which is primary therapy for bipolar disorder and known to induce obesity in humans, inhibited the differentiation of mouse brown adipocytes (Rodríguez de la Concepción et al., 2005). In the sole physiological study, clozapine reversed the thermogensis of brown adipose tissue in rabbits (Blessing et al., 2006). Taken together, these findings suggest that SGA are very likely to induce weight gain by the inhibition of brown adepogenesis. Therefore, we examined the relevance between SGA and weight gain by comparing the effect of clozapine, quetiapine, and ziprasidone on the differentiation of mouse brown preadipocytes. In this study, we provide for the first time biochemical and molecular biological evidence showing that SGA may induce weight gain and metabolic disturbances by inhibiting brown adipocyte differentiation.
Results and Discussion
Rationale for the selection of drugs and their concentration
To reveal the possible involvement of BAT in clozapine- or quetiapine-induced weight gain, we examined the effect of clozapine, quetiapine, and ziprasidone on the brown adipocyte differentiation. For a more rational and objective comparison, we selected the concentration of each drug at which they exert similar clinical efficacies based on the recent therapeutic reference ranges (TRR) recommended by the Arbeitsgemeinschaft fur Neuropshychopharmakologie und Pharmakopsychiatrie (AGNP) (Hiemke et al., 2011). In addition, since a previous report showed that clozapine did not increase intracellular triglyceride content during adipogenic differentiation of human adipose-derived stem cells at 30 µM, which was 16-fold higher than the upper limit concentration of TRR (Sertié et al., 2011), we evaluated the effect of the drugs at the concentrations that were approximately 20-fold higher than the upper limit of TRR, i.e. clozapine (40 µM), quetiapine (30 µM), and ziprasidone (10 µM).
Effects of drugs on the adipogenic differentiation of brown preadipocytes
To disclose the involvement of BAT in three SGA-induced weight gains, the effect of the drugs on the differentiation of the brown preadipocyte cell line was examined. We induced adipogenic differentiation for 8 days in the presence of clozapine (40 µM), quetiapine (30 µM), and ziprasidone (10 µM), and examined intracellular accumulation of triglyceride by Oil Red-O staining. As shown in Figure 1, brown adipocyte differentiation was suppressed almost completely and partly by clozapine and quetiapine, respectively. However, ziprasidone did not show any effect on the differentiation of brown preadipocytes. When we examined the effect on cell viability, clozapine (1.25-40 µM), quetiapine (10-60 µM), and ziprasidone (2-12 µM) did not demonstrate any cytotoxicity during the adipogenic differentiation period (Supplemental Data Figure S1). These results imply that the inhibitory potency of individual drug on brown adipogenesis may be proportionate to its risk for the induction of weight gain and metabolic disturbances. It is noteworthy that clozapine inhibited the differentiation of brown preadipocytes in our results while it did not induce any changes in white preadipocytes (Hu et al., 2010; Sertié et al., 2011). In addition, olanzapine and resperidone, which showed a similar weight gain risk to clozapine and quetiapine, respectively, also induced adipogenesis in 3T3-L1 cells (Yang et al., 2007; Hu et al., 2010). These findings strongly suggest the differential regulatory role of SGA in the differentiation of white and brown preadipocytes. Ziprasidone (0.2 µM) and clozapine (40 µM) were shown to be highly toxic during adipogenic differentiation from adipose-derived stem cells (Sertié et al., 2011), but in our results, the viability of brown preadipocytes was absolutely not affected even at a much higher (ziprasidone, 10 µM) or at the same (clozapine, 40 µM) concentrations (Supplemental Data Figure S1). Although there are significant differences between these two cell types, these findings suggest the possibility that brown preadipocytes may be more resistant to the cytotoxic activity of SGA than white preadipocytes. In accordance with this point of view, Nisoli et al. (2006) reported that white adipocytes are less prone to apoptotic stimuli than brown adipocytes. Further experiments are needed to investigate the differential responses of white and brown (pre)adipocytes to SGA.
Effect of clozapine, quetiapine and ziprasidone on brown adipocyte differentiation. Brown preadipocytes were differentiated with the induction media in the presence of clozapine (40 µM), quetiapine (30 µM), and ziprasidone (10 µM) or control (0.1% DMSO). At day 8, fully differentiated cells were stained with Oil Red-O and photographed by phase-contrast microscopy. Representative data from three independent experiments are shown (Original magnification, 100 ×).
Effects of drugs on the expression of brown adipocyte markers
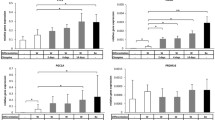
Despite the difference in the developmental origin and physiological function of brown and white adipocytes, both cell types share a very similar transcriptional cascade. Indeed, PPARγ is absolutely necessary for both white and brown fat development (Kajimura et al., 2010). Moreover, C/EBPβ induces PGC-1α (Wang et al., 2008), and has been recently suggested as a critical transcription factor for the initiation of brown fat formation (Kajimura et al., 2009). However, the development of the specific characteristics of brown adipocytes requires the expression of additional transcriptional regulators. PRDM16, a zinc finger protein, activates PPARγ2, forms a transcriptional complex with C/EBPβ (Kajimura et al., 2009), and finally enhances brown adipogenesis by the induction of brown fat-selective markers such as PGC-1α, UCP-1, and Cidea (Kajimura et al., 2009). To provide molecular evidence for our results showing SGA-induced inhibition of brown adipogenesis (Figure 1), we examined the effect of SGA on the mRNA expression of PRDM16, C/EBPβ, PPARγ2, UCP-1, PGC-1α, and Cidea at 8 days after the induction (Figure 2) or during the differentiation of brown preadipocytes (Figure 3, Supplemental Data Figure S2). The results revealed that clozapine significantly inhibited the expression of all genes tested (P < 0.05), and particularly PRDM16, UCP-1 and Cidea were almost disappeared by day 8 (P < 0.05) (Figure 2A). Inhibitory effect of clozapine was time-dependent, and this was apparent from day 4 for PRDM16 and PPARγ2 (Figure 3). Quetiapine also down-regulated the expression of PRDM16, PPARγ2, UCP-1, and Cidea at day 8 after the induction (P < 0.05), but the intensity was much weaker than clozapine (Figure 2B). Inhibitory effect of quetiapine was also not consistent as that of clozapine, for example, CEBPβ was transiently inhibited only at days 4 and 6 (Figure 2C, Supplemental Data Figure S2A). In contrast to clozapine and quetiapine, ziprasidone showed inhibitory activity only in the early stage of the differentiation, and PPARγ2 expression was significantly increased at days 6 and 8 (P < 0.05) (Supplemental Data Figure S2B). These findings clearly correspond with the results from Oil Red-O staining (Figure 1), and strongly support our hypothesis that the inhibition of brown adipogenesis may be one of the mechanisms for SGA-induced weight gain. Among the genes tested in our stuy, transcriptional regulation of PRDM16 by clozapine and quetiapine is remarkable since they are considered as master regulators for brown adipogenesis. Brown adipogenesis from white preadipocytes or myoblasts was successfully induced by the ectopic expression of PRDM16 (Seale et al., 2007), and BAT from PRDM16-/- mice exhibited an abnormal morphology and reduced expression of brown fat markers (Seale et al., 2008). Recently, association of PRDM16 with metabolic syndrome was also suggested (Statistical analysis All values are expressed as the means ± SEM from at least three independent experiments. All statistical calculations were performed using GraphPad Prism 5.03 software (GraphPad Software Inc., San Diego, CA). One- or two-way ANOVA followed by Turkey's Multiple Comparison Test was used to compare means. A probability (P) value less than 0.05 was considered to be significant.
Abbreviations
- ACC:
-
acetyl-CoA carboxylase
- aP2:
-
adipocyte protein 2
- BAT:
-
brown adipose tissue
- CD36:
-
cluster of differentiation 36
- C/EBPβ:
-
CCAAT-enhancer-binding protein beta
- Cidea:
-
cell death-inducing DFFA-like effector a
- CYP:
-
cytochrome P450
- FAS:
-
fatty acid synthase
- GLUT4:
-
glucose transporter 4
- PGC-1α:
-
peroxisome proliferator-activated receptor gamma coactivator-1 alpha
- PKC-β:
-
protein kinase C-beta
- PPARγ:
-
peroxisome proliferator-activated receptor gamma
- PRDM16:
-
PR domain containing 16
- qRT-PCR:
-
quantitative real-time polymerase chain reaction
- SCD1:
-
stearyl-CoA desaturase 1
- SGA:
-
second-generation antipsychotics
- TRR:
-
therapeutic reference ranges
- UCP-1:
-
uncoupling protein-1
References
Bickel MH, Graber BE, Moor M . Distribution of chlorpromazine and imipramine in adipose and other tissues of rats . Life Sci 1983 ; 33 : 2025 - 2031
Birkenaes AB, Opjordsmoen S, Brunborg C, Engh JA, Jonsdottir H, Ringen PA, Simonsen C, Vaskinn A, Birkeland KI, Friis S, Sundet K, Andreassen OA . The level of cardiovascular risk factors in bipolar disorder equals that of schizophrenia: a comparative study . J Clin Psychiatry 2007 ; 68 : 917 - 923
Blessing WW, Zilm A, Ootsuka Y . Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats . Neuroscience 2006 ; 141 : 2067 - 2073
Brandl EJ, Frydrychowicz C, Tiwari AK, Lett TA, Kitzrow W, Büttner S, Ehrlich S, Meltzer HY, Lieberman JA, Kennedy JL, Müller DJ, Puls I . Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain . Prog Neuropsychopharmacol Biol Psychiatry 2012 ; 38 : 134 - 141
Cheng YF, Lundberg T, Bondesson U, Lindström L, Gabrielsson J . Clinical pharmacokinetics of clozapine in chronic schizophrenic patients . Eur J Clin Pharmacol 1988 ; 34 : 445 - 449
Cho YW, Hong S, ** Q, Wang L, Lee JE, Gavrilova O, Ge K . Histone methylation regulator PTIP is required for PPARgamma and C/EBPalpha expression and adipogenesis . Cell Metab 2009 ; 10 : 27 - 39
Deng C, Weston-Green K, Huang XF . The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain ? Prog Neuropsychopharmacol Biol Psychiatry 2010 ; 34 : 1 - 4
Hauner H, Röhrig K, Hebebrand J, Skurk T . No evidence for a direct effect of clozapine on fat-cell formation and production of leptin and other fat-cell-derived factors . Mol Psychiatry 2003 ; 8 : 258 - 259
Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Gründer G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Müller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G . AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011 . Pharmacopsychiatry 2011 ; 44 : 195 - 235
Hu Y, Kutscher E, Davies GE . Berberine inhibits SREBP-1-related clozapine and risperidone induced adipogenesis in 3T3-L1 cells . Phytother Res 2010 ; 24 : 1831 - 1838
Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM . Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex . Nature 2009 ; 460 : 1154 - 1158
Kajimura S, Seale P, Spiegelman BM . Transcriptional control of brown fat development . Cell Metab 2010 ; 11 : 257 - 262
Kraus D, Fasshauer M, Ott V, Meier B, Jost M, Klein HH, Klein J . Leptin secretion and negative autocrine crosstalk with insulin in brown adipocytes . J Endocrinol 2002 ; 175 : 185 - 191
Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL . H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs . Neuropsychopharmacology 2003 ; 28 : 519 - 526
Lane HY, Chang YC, Chang WH, Lin SK, Tseng YT, Jann MW . Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics . J Clin Psychiatry 1999 ; 60 : 36 - 40
Laursen TM, Munk-Olsen T, Nordentoft M, Mortensen PB . Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia . J Clin Psychiatry 2007 ; 68 : 899 - 907
Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Müller DJ . Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications . Mol Psychiatry 2012 ; 17 : 242 - 266
Markowitz JS, Gill HS, DeVane CL, Mintzer JE . Fluoroquinolone inhibition of clozapine metabolism . Am J Psychiatry 1997 ; 154 : 881 -
Matsui-Sakata A, Ohtani H, Sawada Y . Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus . Drug Metab Pharmacokinet 2005 ; 20 : 368 - 378
McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA . Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III . Schizophr Res 2005 ; 80 : 19 - 32
Monteleone P, Fabrazzo M, Tortorella A, La Pia S, Maj M . Pronounced early increase in circulating leptin predicts a lower weight gain during clozapine treatment . J Clin Psychopharmacol 2002 ; 22 : 424 - 426
Nedergaard J, Bengtsson T, Cannon B . Unexpected evidence for active brown adipose tissue in adult humans . Am J Physiol Endocrinol Metab 2007 ; 293 : E444 - E452
Newcomer JW . Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review . CNS Drugs 2005 ; 19 : 1 - 93
Newcomer JW . Metabolic considerations in the use of antipsychotic medications: a review of recent evidence . J Clin Psychiatry 2007 ; 68 : 20 - 27
Nisoli E, Cardile A, Bulbarelli A, Tedesco L, Bracale R, Cozzi V, Morroni M, Cinti S, Valerio A, Carruba MO . White adipocytes are less prone to apoptotic stimuli than brown adipocytes in rodent . Cell Death Differ 2006 ; 13 : 2154 - 2156
Okamatsu-Ogura Y, Nio-Kobayashi J, Iwanaga T, Terao A, Kimura K, Saito M . Possible involvement of uncoupling protein 1 in appetite control by leptin . Exp Biol Med (Maywood) 2011 ; 236 : 1274 - 1281
Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA . Different metabolic responses of human brown adipose tissue to activation by cold and insulin . Cell Metab 2011 ; 14 : 272 - 279
Panariello F, Perruolo G, Cassese A, Giacco F, Botta G, Barbagallo AP, Muscettola G, Beguinot F, Formisano P, de Bartolomeis A . Clozapine impairs insulin action by up-regulating Akt phosphorylation and Ped/Pea-15 protein abundance . J Cell Physiol 2012 ; 227 : 1485 - 1492
Park S, Kim MS, Namkoong C, Park MH, Hong JP . The effect of ziprasidone on body weight and energy expenditure in female rats . Metabolism 2012 ; 61 : 787 - 793
Pavan C, Vindigni V, Michelotto L, Rimessi A, Abatangelo G, Cortivo R, Pinton P, Zavan B . Weight gain related to treatment with atypical antipsychotics is due to activation of PKC-β . Pharmacogenomics J 2010 ; 10 : 408 - 417
Rodríguez de la Concepción ML, Yubero P, Iglesias R, Giralt M, Villarroya F . Lithium inhibits brown adipocyte differentiation . FEBS Lett 2005 ; 579 : 1670 - 1674
Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J . Transient increase in obese gene expression after food intake or insulin administration . Nature 1995 ; 377 : 527 - 529
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM . Transcriptional control of brown fat determination by PRDM16 . Cell Metab 2007 ; 6 : 38 - 54
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM . PRDM16 controls a brown fat/skeletal muscle switch . Nature 2008 ; 454 : 961 - 967
Sertié AL, Suzuki AM, Sertié RA, Andreotti S, Lima FB, Passos-Bueno MR, Gattaz WF . Effects of antipsychotics with different weight gain liabilities on human in vitro models of adipose tissue differentiation and metabolism . Prog Neuropsychopharmacol Biol Psychiatry 2011 ; 35 : 1884 - 1890
Shader RI, Greenblatt DJ . Clozapine and fluvoxamine, a curious complexity . J Clin Psychopharmacol 1998 ; 18 : 101 - 102
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR . New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure . Nature 2008 ; 454 : 1000 - 1004
Van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ . Cold-activated brown adipose tissue in healthy men . N Engl J Med 2009 ; 360 : 1500 - 1508
Viengchareun S, Zennaro MC, Pascual-Le Tallec L, Lombes M . Brown adipocytes are novel sites of expression and regulation of adiponectin and resistin . FEBS Lett 2002 ; 532 : 345 - 350
Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD . Increase in brown adipose tissue activity after weight loss in morbidly obese subjects . J Clin Endocrinol Metab 2012 ; 97 : E1229 - E1233
Wang H, Peiris TH, Mowery A, Le Lay J, Gao Y, Greenbaum LE . CCAAT/enhancer binding protein-beta is a transcriptional regulator of peroxisome-proliferator-activated receptor-gamma coactivator-1alpha in the regenerating liver . Mol Endocrinol 2008 ; 22 : 1596 - 1605
Wang Q, Zhang M, Ning G, Gu W, Su T, Xu M, Li B, Wang W . Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity . PLoS One 2011 ; 6 : e21006
Yang X, Enerbäck S, Smith U . Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance . Obes Res 2003 ; 11 : 1182 - 1191
Yang LH, Chen TM, Yu ST, Chen YH . Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells . Pharmacol Res 2007 ; 56 : 202 - 208
Yang Z, Yin JY, Gong ZC, Huang Q, Chen H, Zhang W, Zhou HH, Liu ZQ . Evidence for an effect of clozapine on the regulation of fat-cell derived factors . Clin Chim Acta 2009 ; 408 : 98 - 104
Zhang JH, Li NF, Yan ZT, Zhang de L, Wang HM, Guo YY, Ling Z . Association of genetic variations of PRDM16 with metabolic syndrome in a general **njiang Uygur population . Endocrine 2012 ; 41 : 539 - 541
Acknowledgements
This research was supported by a grant (10172KFDA993) from Korea Food & Drug Administration in 2012, and by the Catholic Medical Center Research Foundation made in the program year of 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oh, JE., Cho, Y., Kwak, SN. et al. Inhibition of mouse brown adipocyte differentiation by second-generation antipsychotics. Exp Mol Med 44, 545–553 (2012). https://doi.org/10.3858/emm.2012.44.9.062
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2012.44.9.062
- Springer Nature Limited
Keywords
This article is cited by
-
An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications
Reviews in Endocrine and Metabolic Disorders (2023)
-
Lipoxin A4 promotes adipogenic differentiation and browning of mouse embryonic fibroblasts
In Vitro Cellular & Developmental Biology - Animal (2021)
-
Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options
Journal of Endocrinological Investigation (2017)
-
Clozapine modifies the differentiation program of human adipocytes inducing browning
Translational Psychiatry (2016)