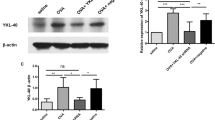
Abstract
To determine the impact of IL-23 knockdown by RNA interference on the development and severity of ovalbumin (OVA)-induced asthmatic inflammation, and the potential mechanisms in mice, the IL-23-specific RNAi-expressing pSRZsi-IL-23p19 plasmid was constructed and inhaled into OVA-sensitized mice before each challenge, as compared with that of control mice treated with alum or budesonide. Inhalation of the pSRZsi-IL-23p19, significantly reduced the levels of OVA-challenge induced IL-23 in the lung tissues by nearly 75%, determined by RT-PCR. In addition, knockdown of IL-23 expression dramatically reduced the numbers of eosinophils and neutrophils in BALF and mitigated inflammation in the lungs of asthmatic mice. Furthermore, knockdown of IL-23 expression significantly decreased the levels of serum IgE, IL-23, IL-17, and IL-4, but not IFNγ, and its anti-inflammatory effects were similar to or better than that of treatment with budesonide in asthmatic mice. Our data support the notion that IL-23 and associated Th17 responses contribute to the pathogenic process of bronchial asthma. Knockdown of IL-23 by RNAi effectively inhibits asthmatic inflammation, which is associated with mitigating the production of IL-17 and IL-4 in asthmatic mice.
Similar content being viewed by others
Introduction
Bronchial asthma is a serious chronic illness with variable clinical symptoms. Because of increased environmental pollution, the incidence of bronchial asthma is increasing worldwide (Eder et al., 2006). Pathogenic studies have revealed that bronchial asthma is characterized by leukocyte infiltration in the bronchial tissues, excessive mucus production, epithelial damage, basement membrane thickening, and smooth muscle hypertrophy in airway epitheial tissues (Barnes, 1989; Boushey and Fahy, 1995). Although many therpaeutic strategies have been utilized for the management of asthmatic patients, the efficacy of these therapies is limited. Hence, development of new effective and safe therapies for the treatment of human asthma will be of great significance.
Interleukin (IL)-23 is a member of the IL-12 heterodimeric cytokine family. IL-23 is composed of p19 and p40, a subunit of IL-12 (Oppmann et al., 2000). IL-23 is a growth factor and inducer of pro-inflammatory Th17 cells, which secrete IL-17 (Plasmid construction Oligonucleotides coding for siRNA that targeted to mouse IL-23p19 (GenBank accession no. NM031252) were designed. The forward and reverse oligonucleotide primers were annealed and ligated into the linearized retroviral vector RNAi-Ready pSIREN-RetroQ-ZsGreen (Clontech, BD Biosciences). The recombinant pSRZSi-IL-23p19 plasmid was double-digested with BamH I and EcoR I restriction enzymes and characterized by 2% agarose gel electrophoresis. The recombinant plasmid pSRZsi-IL-23p19 was further confirmed by sequencing (Applied Biosystem). After that, the identified plasmid was transformed into E. coli and purified with TaKaRa MiNiBEST Plasmid Purification Kit, according to the manufacturers' instruction (TaKaRa Biotechnology). The experimental protocol was approved by the Ethic Committee of our university. Female BALB/c mice at 6-8 weeks of age, weighing 18-22 g, were obtained from the Center of Laboratory Animals, School of Basic Medical Sciences, Jilin University, and maintained at a specific pathogen free facility with a constant humidity and temperature at 12 h/12 h light/dark cycle with free access to food and water. To induce bronchial asthma, the mice were randomized and sensitized by intraperitoneal injection with 10 µg ovalbumin (OVA, grade V, Sigma) and 0.2 mg aluminum hydroxide (Alum) in 100 µl PBS on day 0, 6, and 13. Two weeks after the first immunization, the mice were challenged with aerosolized 1% OVA for 30 min every other day for 5 times over the course of 9 days. Sham groups of mice were injected intraperitoneally with 0.2 mg Alum alone in 100 µl PBS on Day 0, 3, and 6, and were challenged aerosolized 1% OVA for 30 min every other day for 5 times. Another group of mice were inhaled with 2 ml of 50% (1 mg) budesonide pespules (AstraZeneca) for 30 min at 1 h prior to 1% OVA challenges and used as the positive therapeutic controls. The experimental and empty vector groups of mice were inhaled daily with 100 µl of the mixture of 4 µg plasmid or vector DNA and 8 µl Lipofectamine 2000 in 492 µl DMEM for three consecutive days before every sensitization with OVA/Alum. The mice were treated nasally with the same amount of plasmid transfection complex one day prior to 1% OVA challenge. The experimental protocol and manipulation schedule for different groups of mice (n = 10 per group) are illustrated in Figure 1. Twenty-four hours after the final challenge, blood samples were obtained from individual mice for the determination of serum cytokines, and the mice were sacrificed. Their left lungs were collected and fixed in 10% formalin for histological examination. The right lungs of individual mice were frozen at -80℃ for the determination of the relative levels of cytokine mRNA transcripts. The transfection efficiency was determined by visualization of the green fluorescence in the crystal lung sections under a confocal microscope (Olympus, Japan). The relative levels of IL-23 and IL-17 mRNA transcripts in the lung tissues were examined by RT-PCR, using the specific primers (Supplemental data Table S1) on a PTC-100TM thermocycler (MJ. Research). Total RNA was extracted from individual lung tissues by conventional technology, reversely transcribed into cDNA and used as the templates. The PCR reactions were performed in duplicate at 94℃ for 3 min, and subjected to 30 cycles of 94℃ for 30 s, 58℃ for 30 s, 72℃ for 60 s, followed by at 72℃ for 5 min. The GAPDH was used as an internal control with a program of 94℃ for 5 min, 30 cycles of 94℃ for 30 s, 55℃ for 30 s, 72℃ for 60 s, and 72℃ for 5 min. The amplified products were characterized by 1.5% agarose gel electrophoresis and imaged using the gel imaging system (Kodak Digotal Science ID). The formalin-fixed and paraffin-embedded left lung tissues were sectioned at 5 µM and immunostained after deparaffinization and rehydration. The tissue sections were subjected to antigen retrieval and treated with endogenous peroxidase blocking solution. Subsequently, the tissue sections were blocked with goat serum and probed with anti-IL-23 monoclonal antibody or isotype control IgG (4 µg/ml) overnight at 4℃. After washing, the sections were incubated with biotinylated rabbit anti-mouse IgG at room temperature for 20 min and the bound antibodies were detected by peroxidase-conjugated streptavidin, followed by visualizing with DAB. Individual cells with yellow-brown-staining membrane and/or cytoplasm were recognized as positive immunostaining cells. A total of 10 fields were randomly selected from 5 sections of a single mouse under a microscope, and the average number of IL-23-positive cells in individual mice was calculated. In addition, some of the lung tissue sections were stained with hematoxylin and eosin (HE) for characterizing inflammatory infiltrates. The inflammatory scores were graded using a 0-4 grade scoring system (0: no inflammation; 1: mild inflammation; 2: moderate inflammation; 3: severe inflammation; and 4: extreme inflammation), as described previously (Henderson et al., 2002). The BALF was collected from the lungs of individual mice by washing the lungs three times with 0.8 ml of Ca2+- and Mg2+-free cold PBS supplemented with 0.1% BSA and 0.05 mM EDTA. After that, the BALF was centrifuged, and the contained cells were stained with Wright-Giemsa. The frequency of eosinophils in individual BALF samples was counted with a hemocytometer. The frequency of neutrophils was determined in the HE-stained sections. The numbers of eosinophils and neutrophils were expressed over the 1,000 cells in each field, and at least four fields under a phase contrast microscope were recorded for data analysis. Individual sera were prepared by centrifugation and the levels of serum IL-23, IL-17, IL-4, IFN-γ, and IgE were determined by ELISA using the specific kits, according to the manufacturers' instructions. Data are presented as mean ± SD. The difference among different groups was determined by ANOVA and between the two groups was analyzed by F-test or q test. Statistical analyses were performed using SPSS 16.0 statistics software (SPSS Inc., Chicago, IL). A P value of < 0.01 was considered statistically significant.Mice and induction of asthma
RT-PCR
Immunohistological and immunohistochemistric analysis
Characterization of lung morphology and leukocytes in blood, tissue, and bronchoalveolar lavage fluid (BALF)
ELISA
Statistical analysis
Abbreviations
- Alum:
-
aluminum hydroxide
- BALF:
-
bronchoalveolar lavage fluid
- COPD:
-
chronic obstructive pulmonary disease
- CIA:
-
collagen-induced arthritis
- DAB:
-
diaminobenzidine
- EAE:
-
encephalomyelitis
- HE:
-
hematoxylin and eosin
- IBD:
-
inflammatory bowel disease
- OVA:
-
ovalbumin
- RT-PCR:
-
reverse transcription polymerase chain reaction
- RNAi:
-
RNA interference
- VCAM-1:
-
vascular cell adhesion molecule
- VLA4:
-
very late antigen-4
References
Aggarwal S, Ghilardi N, **e MH, de Sauvage FJ, Gurney AL . Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17 . J Biol Chem 2003 ; 278 : 1910 - 1914
Barczyk AW, Pierzchala W, Sozañska E . Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine . Respir Med 2003 ; 97 : 726 - 733
Barnes PJ . New concepts in the pathogenesis of bronchial hyperresponsiveness and asthma . J Allergy Clin Immunol 1989 ; 83 : 1013 - 1026
Boushey HA, Fahy JV . Basic mechanisms of asthma . Environ Health Perspect 1995 ; 103 : 229 - 233
Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H . Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma . Eur J Immunol 1998 ; 28 : 3240 - 3251
Eder W, Ege MJ, von Mutius E . The asthma epidemic . N Engl J Med 2006 ; 355 : 2226 - 2235
Fahy JV, Liu J, Wong H, Boushey HA . Analysis of cellular and biochemical constituents of induced sputum after allergen challenge: a method for studying allergic airway inflammation . J Allergy Clin Immunol 1994 ; 93 : 1031 - 1039
Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ . Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice . J Immunol 2004 ; 172 : 2827 - 2833
Henderson WR, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY . A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model . Am J Respir Crit Care Med 2002 ; 165 : 108 - 116
Hopfenspirger MT, Parr SK, Hopp RJ, Townley RG, Agrawal DK . Mycobacterial antigens attenuate late phase response, airway hyperresponsiveness, and bronchoalveolar lavage eosinophilia in a mouse model of bronchial asthma . Int Immunopharmacol 2001 ; 1 : 1743 - 1751
Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhäll C, Sjöstrand M, Kolls JK, Anderson GP, Lindén A . Functional relevance of the IL-23-IL-17 axis in lungs in vivo . Am J Respir Cell Mol Biol 2007 ; 36 : 442 - 451
Koo GC, Shah K, Ding GJ, **ao J, Wnek R, Doherty G, Tong XC, Pepinsky RB, Lin KC, Hagmann WK, Kawka D, Singer II . A small molecule very late antigen-4 antagonist can inhibit ovalbumin-induced lung inflammation . Am J Respir Crit Care Med 2003 ; 167 : 1400 - 1409
McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK . Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis . J Immunol 2005 ; 175 : 404 - 412
McKenzie BS, Kastelein RA, Cua DJ . Understanding the IL-23-IL-17 immune pathway . Trends Immunol 2006 ; 27 : 17 - 23
Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fu**o M . Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma . Proc Natl Acad Sci USA 2001 ; 98 : 5175 - 5180
Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA . Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12 . Immunity 2000 ; 13 : 715 - 725
Peachell P . Targeting the mast cell in asthma . Curr Opin Pharmacol 2005 ; 5 : 251 - 256
Peng J, Yang XO, Chang SH, Yang J, Dong C . IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation . Cell Res 2010 ; 20 : 62 - 71
Thompson AB, Daughton D, Robbins RA, Ghafouri MA, Oehlerking M, Rennard SI . Intraluminal airway inflammation in chronic bronchitis. Characterization and correlation with clinical parameters . Am Rev Respir Dis 1989 ; 140 : 1527 - 1537
Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H . IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice . Am J Respir Crit Care Med 2008 ; 178 : 1023 - 1032
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Experimental & Molecular Medicine website
Supplementary information
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, Y., Sun, M., Cheng, H. et al. Silencing IL-23 expression by a small hairpin RNA protects against asthma in mice. Exp Mol Med 43, 197–204 (2011). https://doi.org/10.3858/emm.2011.43.4.024
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2011.43.4.024
- Springer Nature Limited
Keywords
This article is cited by
-
Schizophyllum commune induces IL-17-mediated neutrophilic airway inflammation in OVA-induced asthma model mice
Scientific Reports (2019)
-
Eosinophils: changing perspectives in health and disease
Nature Reviews Immunology (2013)
-
The potential of biologics for the treatment of asthma
Nature Reviews Drug Discovery (2012)
-
Treatment of allergic asthma: Modulation of Th2 cells and their responses
Respiratory Research (2011)