Abstract
Previous research suggests that excessive negative self-related thought during mind wandering involves the default mode network (DMN) core subsystem and the orbitofrontal cortex (OFC). Heart rate variability (HRV) biofeedback, which involves slow paced breathing to increase HRV, is known to promote emotional well-being. However, it remains unclear whether it has positive effects on mind wandering and associated brain function. We conducted a study where young adults were randomly assigned to one of two 5-week interventions involving daily biofeedback that either increased heart rate oscillations via slow paced breathing (Osc+ condition) or had little effect on heart rate oscillations (active control or Osc- condition). The two intervention conditions did not differentially affect mind wandering and DMN core-OFC functional connectivity. However, the magnitude of participants’ heart rate oscillations during daily biofeedback practice was associated with pre-to-post decreases in mind wandering and in DMN core-OFC functional connectivity. Furthermore, the reduction in the DMN core-OFC connectivity was associated with a decrease in mind wandering. Our results suggested that daily sessions involving high amplitude heart rate oscillations may help reduce negative mind wandering and associated brain function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mind wandering involves generating thoughts unrelated to the present situation. While it is not inherently detrimental (Konjedi & Maleeh, 2017; Poerio et al., 2013) and can be beneficial in some situations (e.g., creative thinking, problem solving, and constructive positive daydreaming; Baird et al., 2012; Gable, Hopper, & Schooler, 2019; McMillan, Kaufman, & Singer, 2013), emotional consequences of mind wandering tend to be more negative than positive. In one study (Killingsworth & Gilbert, 2010), 2,250 adults of various ages, occupations, and nationalities answered the following questions about their daily activities via a web application: “How are you feeling right now?”; “What are you doing right now?”; and “Are you thinking about something other than what you’re currently doing?” The results revealed that mind wandering occurred in 46.9% of their samples and that people reported being less happy when their minds were wandering than when they were not.
During mind wandering, people often experience thoughts and feelings about themselves and their life (D’Argembeau, 2018). The default mode network (DMN) is a brain network involved in both mind wandering and self-related thoughts ( Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Mason et al., 2007). Although the DMN was originally regarded as a unitary system, more recent research suggested that the DMN can be divided into three functionally independent subsystems, namely the core, medial temporal lobe, and dorsal medial prefrontal cortex subsystems (Andrews-Hanna, 2012; Andrews-Hanna et al., 2010, 2014). The core subsystem, which consists of anterior medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC), is particularly important for self-referential processing (Andrews-Hanna, 2012; Andrews-Hanna et al., 2010) and also is implicated in rumination and depression (Burkhouse et al., 2017; Cooney et al., 2010; Zhou et al., 2020). In depressed patients, self-generated thoughts were more negative, less positive, more self-related, and more past-related, relative to healthy controls (Hoffmann et al., 2016). Another key region associated with depression and other psychological disorders is the orbitofrontal cortex (OFC; Cooney et al., 2010; Drevets, 2007; Rolls, 2019; Rolls et al., 2020), which is involved in emotion-related learning and decision-making, rewards, punishments, and emotional and behavioral flexibility (O'Doherty et al., 2001; Rempel-Clower, 2007; Rolls, 2004, 2019). Functional and structural alterations in the OFC occur in many psychiatric disorders, including anxiety disorders, major depression, posttraumatic stress disorder, obsessive–compulsive disorder, schizophrenia, bipolar, and personality disorders (Jackowski et al., 2012; Milad & Rauch, 2007; Thorsen et al., 2018). Importantly, previous studies found that depression increased resting state functional connectivity between the lateral OFC and the PCC/precuneus (part of the DMN core subsystem; Cheng et al., 2022) found that functional connectivity between the PCC and the medial OFC plays a role in negative spontaneous nonintentional thoughts (or negative mind wandering). Although the relationship between DMN-OFC hyperconnectivity and mind wandering is a relatively new area of investigation, previous studies together suggest that the interaction between the DMN core subsystem and the OFC may play a key role in excessive negative self-related thought during mind wandering.
Meditation can help reduce episodes of mind wandering and promote emotional well-being (Brandmeyer & Delorme, 2018; Mrazek et al., 2013; Rodriguez-Larios et al., 2021; van Agteren et al., 2021). Meditation also can decrease activity in the primary regions of the DMN (the mPFC and PCC/precuneus) during meditation relative to resting state (Brewer et al., 2011), during meditation relative to an active cognitive task (Garrison et al., 2015), and during present-centered self-focus versus narrative self-focus (Farb et al., 2007). Some meditative practices not only improve psychological health but also have a positive impact on physiological well-being, such as slowing down breathing and increasing heart rate variability (HRV; Bernardi et al., 2001; Lehrer et al., 1999; Peng et al., 1999, 2004; Phongsuphap et al., 2008). During these meditative practices, breathing slows down and drives large heart rate oscillations at the breathing frequency (Bernardi et al., 2001; Lehrer et al., 1999; Peng et al., 2004). Prior research suggests that slow-paced breathing practices alone (without explicit meditation training) can improve emotional well-being. Using an HRV biofeedback procedure, participants can be trained to breathe at their resonance frequency, which is around 0.1 Hz or 10 s per breath (Lehrer et al., 2013). During paced breathing at their own baroreflex resonance frequency, people can significantly increase acute levels of HRV. Repeated daily practice over several weeks promote long-term emotional health benefits (for review see Lehrer & Gevirtz, 2014). Recent meta analyses (Lehrer et al., 2020; Pizzoli et al., 2021) also suggested that HRV biofeedback improves symptoms of various types of psychological disorders, such as depression and anxiety, in both clinical and nonclinical populations. Consistent with these prior emotion-related effects, our recent randomized clinical trial (for preprint see Nashiro et al., 2021) demonstrated that a 5-week intervention involving daily biofeedback aimed at increasing heart rate oscillations significantly increased resting-state functional connectivity in emotion-related networks, whereas it did not have a significant impact on functional connectivity in the overall DMN.
It remains unclear whether the HRV biofeedback has positive effects on mind wandering and associated brain function. To address these questions, we used the data from our previous study where 106 healthy younger adults were randomly assigned to one of two 5-week interventions involving daily biofeedback that either increased heart rate oscillations (Osc+) or had little effect on heart rate oscillations (Osc−). We formulated this question after initiating our trial and so added a measure of mind wandering after the study started; about half of the participants completed the measure (N = 55). We tested the hypotheses that the Osc+ intervention would reduce the tendency for mind wandering and decrease functional connectivity between the DMN core subsystem and the OFC, relative to the Osc− intervention.
Methods
Participants
We recruited 121 participants aged between 18 and 35 years through a USC online bulletin board, the USC Healthy Minds community subject pool, Facebook, and flyers. Participants signed informed consent approved by the University of Southern California (USC) Institutional Review Board. Prospective participants were screened and excluded for major medical, neurological, or psychiatric illnesses. We also excluded people who had a disorder that would impede performing the HRV biofeedback procedures (e.g., coronary artery disease, angina, cardiac pacemaker), who currently trained in relaxation, biofeedback or breathing techniques, or were on any psychoactive drugs other than antidepressants or antianxiety medications. We included people who were taking antidepressant or antianxiety medication and/or attending psychotherapy only if the treatment had been ongoing and unchanged for at least 3 months and no changes were anticipated. Eligible participants were assigned to small groups of 3–6 people, and each group met at the same time and day each week except for the weeks when they underwent MRI. After recruitment and scheduling of each wave of groups were complete, we randomly assigned groups to a condition by using flip** a coin method. Hence, each participant was randomly assigned to one condition involving daily biofeedback aimed at increasing heart rate oscillations (Osc+) or decreasing heart rate oscillations (Osc-). Upon completing the study, participants were paid for their participation and received bonus payments based on their individual and group performances (see “Rewards for Performance” in Supplementary Materials for more details). Of 121 participants, 106 completed the interventions, and 100 completed the resting state scan both before and after the intervention. Two participants (one person from each condition) were excluded due to unsuccessful denoising pipeline results. Additionally, two Osc+ participants who did not follow the instructions were excluded from the analyses (a predefined exclusion criterion). During the postintervention resting state scan, these two participants breathed slowly as if they were engaged in the Osc+ biofeedback (i.e., they failed to follow the instructions, which were to simply rest while looking at the white cross at the center of the screen and breathing normally). The remaining 96 participants were included in the resting state analyses (Supplemental Fig. 1; Supplementary Table 1). For the analyses involving heart rate data, three participants in the Osc− condition were excluded, because we failed to save heart rate data from ear sensors due to technical issues with the first version of the Osc− biofeedback software, leaving 93 of 96 participants in the analyses. For the analyses involving behavioral data (i.e., the mind wandering score detailed in the “Behavioral Assessments” section), we used data from a subset of 55 participants, because the measure was added after initiation of the study protocol.
Overview of 7-week protocol schedule
A full description of the study has been detailed in a preprint (Nashiro et al., 2021). Briefly, the study protocol involved seven weekly lab visits and 5 weeks of home biofeedback training. The first lab visit involved the non-MRI baseline measurements, including emotion questionnaires. The second lab visit involved baseline MRI assessments followed by the first biofeedback training session. Between the second and seventh lab visits (approximately 5 weeks), participants were instructed to engage in daily biofeedback practice 20–40 min/day and visit the lab weekly. The sixth lab visit repeated the emotion questionnaires from the first lab visit. The seventh lab visit repeated the baseline MRI assessments.
Biofeedback training
Osc+ condition
In the second lab visit, Osc+ participants tried out several breathing paces around 10 s/breath to see which induced the largest oscillations in their heart rate (their own resonance frequency; Lehrer et al., 2013) using the emWave Pro software (Heartmath, 2016). Participants were asked to wear an ear sensor to measure their pulse and to breathe in and out with a visual pacer. They were instructed to inhale through the nose when the blue bar went up and to exhale through the mouth when the blue bar went down (e.g., 5-s inhale/5-s exhale for 10 s/breath cycle). They were asked to breathe at 5 different paces (9 s, 10 s, 11 s, 12 s, and 13 s per breath, which approximately corresponds with 6.5, 6, 5.5, 5, and 4.5 breaths per minute as in Lehrer et al. (2013) for 5 min each. To identify each participant’s best approximate resonance frequency, researchers evaluated various aspects of the oscillatory dynamics for each breathing pace using Kubios HRV Premium 3.1 software (Tarvainen et al., 2014) and assessed which one had the most of the following characteristics: highest low frequency (LF) power, the highest maximum LF amplitude peak on the spectral graph, highest peak-to-trough amplitude, cleanest and highest-amplitude LF peak, highest coherence score, and highest root mean square of successive differences (RMSSD).
To complete home training, participants received an ear sensor and a small laptop with the emWave Pro software. During their daily training sessions, participants breathed to a pacer set to their resonance frequency determined in their prior lab visit. They received biofeedback on their heart rate oscillatory activity via a real-time plot of their heart rate and a “coherence” score, which is a built-in metric score provided by the emWave Pro software. Coherence is characterized by a sine-wave-like pattern in the HRV waveform in the LF range (McCraty et al., 2009). Thus, a higher coherence score is reflected in the HRV power spectrum as a large increase in power in the LF band around 0.1 Hz. Participants were instructed to aim for a high coherence score. The coherence score was calculated as peak power/(total power – peak power). Peak power was identified by finding the highest peak within the range of 0.04–0.26 Hz and calculating the integral of the window 0.015 Hz above and below this highest peak. Total power was computed for the 0.0033–0.4 Hz range.
In the third week, participants returned to the lab to receive coaching from researchers who checked again which breathing frequency produced the strongest heart rate oscillations (i.e., resonance frequency). Participants were asked to breathe at 3 different paces for 5 min each: the best pace from the prior week’s visit, a one second per breath shorter, and a one second per breath longer than their best pace (e.g., if their best pace in the prior week was 13 s, they tried 12 s, 13 s, and 14 s per breath). As in the second week, researchers evaluated the same aforementioned characteristics of these three paces and assigned the participant the one that best approximated their resonance frequency for home training that week. In subsequent weekly visits, during 5-min training segments, participants were asked to try out abdominal breathing with pursed lips as well as other strategies of their choice to enhance coherence in a relaxed manner (Lehrer et al., 2013). Their assigned breathing paces ranged from 9–14 s/breath. The mean was 11.16 s (SD = 1.49) and the median was 11 s.
Osc− condition
An ideal comparison to this Osc+ condition would be another condition with similar biofeedback information, participant expectations and time spent training but no increases in heart rate oscillatory activity during the training sessions. Thus, we designed a decrease-oscillations comparison condition (Osc−) in which participants received heart rate biofeedback designed to reduce their heart rate oscillations while breathing normally. In addition, to try to avoid having them discover that they could reduce HRV simply by increasing physical activity (Sarmiento et al., 2013), we instructed them also to try to decrease their heart rate during the training sessions.
In the second lab visit, participants were asked to think of five strategies to lower heart rate and heart rate oscillations (e.g., imaging the ocean, listening to nature sounds, listening to instrumental music, thinking of their loved ones, occasionally closing eyes). Participants were asked to wear the same biofeedback ear sensor as Osc+ participants and view real-time heart rate biofeedback while they tried each strategy for 5 min. Researchers analyzed the data using Kubios HRV Premium 3.1 software and identified the best strategy as the one that exhibited the opposite characteristics from the Osc+ condition: lowest LF power, the minimum LF amplitude peak on the spectral graph, lowest peak-to- trough amplitude, multiple and lowest-amplitude LF peak, highest calmness score (see below for more details), and lowest RMSSD.
To complete home training, participants received an ear sensor and a small laptop with a custom software. During their daily training sessions, participants aimed at reducing heart rate and heart rate oscillations using the best strategy determined in their prior lab visit. In contrast with the Osc+ group, the custom-developed software provided a “calmness” score, which was calculated by multiplying the coherence score that would have been displayed in the Osc+ condition by − 1 and adding 10 (i.e., “anti-coherence” score). The net result was that participants got more positive feedback and higher calmness scores when their heart rate oscillatory activity in the 0.04 – 0.26 Hz range was low (see “Osc− condition” in Supplementary Materials for more details).
In the third lab visit, participants were asked to select three strategies and try them for 5 min each. The strategy identified as best (based on the aforementioned characteristics) was selected as the one to focus on during home training sessions the following week. In subsequent weekly visits, during 5-min training segments, participants were again asked to try out strategies of their choice.
Brain imaging data acquisition and preprocessing
We employed a 3 T Siemens MAGNETOM Trio scanner with a 32-channel head array coil at the USC Dana and David Dornsife Neuroimaging Center. T1-weighted 3D structural MRI brain scans were acquired pre- and post-intervention using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence with TR = 2,300 ms, TE = 2.26 ms, slice thickness = 1.0 mm, flip angle = 9°, field of view = 256 mm, and voxel size = 1.0 × 1.0 × 1.0 mm, with 175 volumes collected (4:44 min). Resting state functional MRI was acquired using multi-echo-planar imaging sequence with TR = 2,400 mm, TE 18/35/53 ms, slice thickness = 3.0 mm, flip angle = 75°, field of view = 240 mm, voxel size = 3.0 × 3.0 × 3.0 mm, 175 volumes, and acquisition time = 7 min. During the resting state scan, participants were instructed to rest, breathe normally, and look at the central white cross on the black screen.
To minimize the effects of motion and physiological effects, we used multi-echo sequences during our fMRI scans. Previous research suggests that Blood Oxygen Level Dependent (BOLD) T2* signal is linearly dependent on echo time, whereas non-BOLD signal is not echo-time dependent (Kundu et al., 2012). Multi-echo acquisitions allow uncoupling of BOLD signal from movement artifact and significantly improve accuracy of functional connectivity analyses (Dipasquale et al., 2017). Thus, we implemented a denoising pipeline using independent components analysis (ICA) and echo-time dependence to distinguish BOLD fluctuations from non-BOLD artifacts, including motion and physiology (Kundu et al., 2013). In addition, the six motion parameters and signal from white matter and cerebrospinal fluid were removed from each participant’s preprocessed data. We applied a band-pass filter of 0.01–0.1 Hz and used FSL FLIRT to linearly align the denoised data to each participant’s brain-extracted structural image and the standard MNI 2-mm brain.
Heart rate data acquisition and preprocessing
Kubios HRV Premium 3.1 was used to compute autoregressive spectral power for each training session over the course of 5 weeks. We averaged the autoregressive total spectral power from all training sessions for each participant. We extracted the summed power within the 0.063 ~ 0.125 Hz range for each participant (corresponding with 8–16 s, a range encompassing breathing paces used by Osc+ participants) to obtain a measure of resonance frequency oscillatory activity during biofeedback. Before conducting statistical analyses, we log transformed the power values.
Behavioral assessments
At pre- and post-intervention lab visits (the first and sixth visits), a subset of 55 participants completed the 20-item version of the Five Facet Mindfulness Questionnaire (FFMQ). The FFMQ consisted of five subscales: acting with awareness, observing, describing, nonjudging of inner experience, and nonreacting of inner experience. In this study, we were particularly interested in the acting with awareness subscale, which measures the tendency to get distracted from the present-moment experience by mind wandering. Items for this subscale include, “when I do things, my mind wanders off and I’m easily distracted,” “I don’t pay attention to what I’m doing because I’m daydreaming, worrying, or otherwise distracted,” “I’m easily distracted,” and “I find it difficult to stay focused on what’s happening in the present.” Participants responded to each item using a 5-point scale (1 = Never/rarely true and 5 = Very often/always true). Previous research found that the acting with awareness subscale is associated with spontaneous mind wandering (Seli et al., 2015) and the frequency of mind wandering episodes in daily life (Ottaviani & Couyoumdjian, 2013). The items in the acting with awareness subscale is usually reverse scored; however, for the ease of the readers, we used the nonreversed scores with higher scores indicating greater tendency for mind wandering (hereinafter called “the mind wandering score”).
Analyses
Brain data analysis
We used the DMN core subsystem and OFC masks defined in a prior study (Thomas Yeo et al., 2011). We performed a FSL dual-regression analysis using the DMN core mask as the ROI, which created subject-specific time series for the DMN core regions. The resulting individual time series were used to create subject-specific spatial maps of the DMN core ROI. From the subject-specific z-transformed maps, we extracted mean functional connectivity values from the OFC, which represent functional connectivity strengths between the DMN core regions and OFC. We calculated the difference between pre and post functional connectivity values by subtracting pre values from post values. We conducted independent t-tests to examine whether the Osc+ and Osc− conditions differ in pre-post changes in functional connectivity. The difference score also was used in correlation analyses.
HRV data analysis
For each participant, we calculated the average heart rate oscillation power within the resonance frequency range of 0.063 ~ 0.125 Hz during home training across the 5-week training period (hereinafter called “training oscillation power”), which was used as the training performance index in the analyses. We performed an independent t-test to examine whether the two conditions differ in training oscillation power. The training oscillation power also was used in correlation analyses.
Behavioral data analysis
We summed the four items in the acting with awareness subscale (i.e., the mind wandering score). Higher scores indicate greater tendency for mind wandering. We then computed the difference between pre and post mind wandering scores by subtracting pre values from post values. We performed an independent t-test to examine whether the two conditions differ in pre-post changes in the mind wandering score. The difference score also was used in correlation analyses.
Results
As a manipulation check, we examined whether the participants in the two conditions differed in training oscillation power. Consistent with our prior report with a larger sample size (for preprint see Nashiro et al., 2021), there was a significant condition difference in training oscillation power (see Supplemental Table 2 for the values). We also report condition differences in other HRV indices during training and baseline in Supplemental Table 2.
We examined whether the two conditions differ in pre-to-post changes in functional connectivity between the DMN core subsystem and the OFC (Core-OFC). There was no significant condition difference, t(94) = − 1.12, p = 0.264, r = 0.114 (Supplementary Fig. 2). We also examined whether the two conditions differ in pre-to-post changes in mind wandering, but no significant difference was found, t(53) = − 0.45, p = 0.654, r = 0.061 (Supplementary Fig. 3). Additionally, we performed 2 (timepoint: pre vs. post) × 2 (condition: Osc+ vs. Osc−) ANOVAs for Core-OFC functional connectivity and mind wandering; however, no significant results were found (Supplementary Fig. 4).
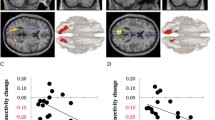
Next, we examined whether training oscillation power (i.e., training performance index) was associated with pre-to-post changes in functional connectivity across all participants in both conditions. There was a significant negative correlation between training oscillation power and pre-post change in Core-OFC connectivity, r(91) = − 0.299, p = 0.004, CI [− 0.474, − 0.102] (Fig. 1), such that higher training oscillation power was associated with a greater reduction in Core-OFC functional connectivity. Separate analyses for each condition also showed a significant negative correlation between training oscillation power and pre-post change in Core-OFC connectivity for the Osc+ condition, r(47) = − 0.343, p = 0.016, CI [− 0.570, − 0.069], and for the Osc− condition, r(42) = − 0.330, p = 0.029, CI [− 0.571, − 0.037].
Correlation between training oscillation power and pre-post change in DMN Core-OFC functional connectivity. Greater training oscillation power was associated with a greater reduction in DMN Core-OFC functional connectivity across both conditions. When we removed two people whose standard deviation was greater than 2.5 for post–pre Core-OFC connectivity, the correlation remained significant, r(89) = − 0.269, p = 0.010, CI [− 0.450, − 0.066]
There was a negative correlation between training oscillation power and pre-post change in mind wandering scores, r(51) = − 0.322, p = 0.019, CI [− 0.545, − 0.056] (Fig. 2), suggesting that higher training oscillation power was associated with a greater reduction in mind wandering. The confidence interval (CI) indicates that the correlation could be as small as − 0.056, which would not be very meaningful, to as high as − 0.545, which would be quite meaningful. Our data are consistent with either of these interpretations, and a larger sample size would increase the precision (decrease the CI). In addition, a greater reduction in mind wandering was associated with a greater decrease in Core-OFC connectivity, r(51) = 0.304, p = 0.027, CI [0.037, 0.531] (Fig. 3). The CI indicates that the correlation could be as small as 0.037, which would not be very meaningful to as high as 0.531, which would be quite meaningful.
Correlation between training oscillation power and pre-post change in mind wandering. Greater training oscillation power was associated with a greater reduction in mind wandering across both conditions. When we removed one person whose standard deviation was greater than 2.5 for post–pre mind wandering score, the correlation remained significant, r(50) = − 0.331, p = 0.016, CI [− 0.554, − 0.064]
Discussion
The two interventions did not significantly differ in pre-post changes in Core–OFC functional connectivity and mind wandering. However, higher training oscillation power (i.e., how large participants’ heart rate oscillations were during daily training) was associated with decreases in Core–OFC functional connectivity and mind wandering. Furthermore, reduced Core–OFC connectivity was associated with a decrease in mind wandering.
Previous research suggests that greater Core-OFC functional connectivity is associated with excessive negative self-related thought during mind wandering. Our results suggested that daily sessions involving high amplitude heart rate oscillations may help to reduce both Core-OFC functional connectivity and mind wandering beyond the session time itself. The current findings also provide some insights into a possible link between HRV, DMN, and mind wandering. In the neurovisceral integration model (Thayer & Lane, 2000, 2009), it was proposed that HRV reflects the activity of an integrative neural network regulating physiological, emotional, and cognitive responses. In this model, the prefrontal cortex (including the mPFC that is part of the DMN core subsystem) exerts inhibitory control over subcortical regions. Higher resting HRV reflects more effective prefrontal cortex ability to inhibit subcortical circuits (Thayer & Lane, 2000, 2009) and thus is associated with better emotion regulation (Williams et al., 2015). However, HRV may go beyond signaling the functioning of regulatory brain regions, that is, inducing high HRV may improve the brain’s capacity to regulate emotion (Mather & Thayer, 2018). In fact, our recent randomized clinical trial (for preprint see Nashiro et al., 2021), which includes the participants in the present study, demonstrated that daily sessions inducing high heart rate oscillations significantly increased resting-state functional connectivity within emotion-related networks involving a wide range of emotional and autonomic processes. Together with our current findings, it seems plausible that inducing large heart rate oscillations increases brain’s regulatory activity while decreasing brain’s maladaptive activity (e.g., DMN Core-OFC hyperconnectivity). Such alterations of emotion-related brain networks may contribute to improving emotional well-being by diminishing negative mind wandering.
As discussed earlier, meditation also helps to reduce mind wandering (Brandmeyer & Delorme, 2018; Mrazek et al., 2013; Rodriguez-Larios et al., 2021) and DMN activation (Brewer et al., 2011; Farb et al., 2007; Garrison et al., 2015). Some meditative practices also slow down breathing and increase heart rate variability (Bernardi et al., 2001; Lehrer et al., 1999; Peng et al., 1999; Phongsuphap et al., 2008). Thus, these physiological changes (i.e., slow breathing and large heart rate oscillations) are commonly seen during some meditative practices and HRV biofeedback training, both of which are known to improve emotional well-being (Goyal et al., 2014; Khoury et al., 2017; Lehrer et al., 2020; Pizzoli et al., 2021). Although the underlying mechanism is still unclear, physiological changes during meditation may contribute to emotional well-being. It is possible that meditative practices that incorporate high amplitude heart rate oscillations may be more effective in reducing mind wandering and associated brain function than meditations that do not integrate such physiological changes.
Some limitations should be noted. There is some debate in the literature about how to conceptualize mind wandering. There are several definitions of mind wandering, such as task-unrelated thought, stimulus-independent thought, and spontaneous versus deliberate thought (Christoff et al., 2016; Seli et al., 2018; Smallwood & Schooler, 2015). Some researchers argue that mind wandering is a heterogeneous construct with multiple facets instead of a unitary construct (Seli et al., 2018). In this study, we used the acting of awareness subscale as a measure of mind wandering in everyday life. Thus, it is possible that we failed to capture specific aspects of mind wandering, such as task-related versus task-unrelated thought (Smallwood & Schooler, 2015) and spontaneous versus deliberate mind wandering (Seli et al., 2015). An important area of future research is to investigate how different domains of mind wandering may be influenced by high amplitude of heart rate oscillations. Another limitation of this study is that only a subset of participants completed the mind-wandering questionnaire, because it was added after initiation of the study protocol. Future studies should replicate the current findings with a larger sample size in order to increase the precision of a confidence interval (Amrhein et al., 2019) although it is important to note that a low sample size in the current study cannot lead to a spurious significant effect (Zuckerman et al., 1993). It would be worthwhile for future research to further investigate neutral mechanisms linking HRV and mind wandering. In addition, future studies should investigate common and separate neural mechanisms underlying heart rate oscillation intervention and meditation practices and how they jointly or separately promote emotional well-being (e.g., testing whether attention to the breath during meditation practices vs. Osc+ training leads to similar or different neural patterns and behavioral outcomes; Weng et al., 2020). It also is important to replicate the current findings in clinical populations, including individuals with depression and anxiety disorders.
Conflict of interest
The authors declare no competing interests.
Open practices statement
Data supporting the findings of this study will be made publicly available at OpenNeuro. None of the present analyses were preregistered; however, the original study was preregistered (ClinicalTrials.gov NCT03458910; Heart Rate Variability and Emotion Regulation or “HRV-ER”).
References
Amrhein, V., Greenland, S., & McShane, B. (2019). Comment: Retire statistical significance. Nature, 567, 305–307.
Andrews-Hanna, J. R. (2012). The brain’s default network and its adaptive role in internal mentation. The Neuroscientist, 18(3), 251–270.
Andrews-Hanna, J. R., Reidler, J. S., Sepulcre, J., Poulin, R., & Buckner, R. L. (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562.
Andrews-Hanna, J. R., Saxe, R., & Yarkoni, T. (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage, 91, 324–335.
Baird, B., Smallwood, J., Mrazek, M. D., Kam, J. W., Franklin, M. S., & Schooler, J. W. (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122.
Bernardi, L., Sleight, P., Bandinelli, G., Cencetti, S., Fattorini, L., Wdowczyc-Szulc, J., & Lagi, A. (2001). Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: Comparative study. BMJ, 323(7327), 1446–1449.
Brandmeyer, T., & Delorme, A. (2018). Reduced mind wandering in experienced meditators and associated EEG correlates. Experimental Brain Research, 236(9), 2519–2528.
Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang, Y.-Y., Weber, J., & Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences, 108(50), 20254–20259.
Burkhouse, K. L., Jacobs, R. H., Peters, A. T., Ajilore, O., Watkins, E. R., & Langenecker, S. A. (2017). Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cognitive, Affective, & Behavioral Neuroscience, 17(2), 394–405.
Cheng, W., Rolls, E. T., Qiu, J., **e, X., Wei, D., Huang, C.-C., Yang, A. C., Tsai, S.-J., Li, Q., & Meng, J. (2018). Increased functional connectivity of the posterior cingulate cortex with the lateral orbitofrontal cortex in depression. Translational Psychiatry, 8(1), 1–10.
Christoff, K., Gordon, A. M., Smallwood, J., Smith, R., & Schooler, J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, 106(21), 8719–8724.
Christoff, K., Irving, Z. C., Fox, K. C., Spreng, R. N., & Andrews-Hanna, J. R. (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731.
Cooney, R. E., Joormann, J., Eugène, F., Dennis, E. L., & Gotlib, I. H. (2010). Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience, 10(4), 470–478.
D’Argembeau, A. (2018). Mind-wandering and self-referential thought. The Oxford handbook of spontaneous thought: Mind-wandering, creativity, and dreaming, 181–191.
Dipasquale, O., Sethi, A., Laganà, M. M., Baglio, F., Baselli, G., Kundu, P., Harrison, N. A., & Cercignani, M. (2017). Comparing resting state fMRI de-noising approaches using multi-and single-echo acquisitions. PloS One, 12(3).
Drevets, W. C. (2007). Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences, 1121(1), 499–527.
Farb, N. A., Segal, Z. V., Mayberg, H., Bean, J., McKeon, D., Fatima, Z., & Anderson, A. K. (2007). Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience, 2(4), 313–322.
Gable, S. L., Hopper, E. A., & Schooler, J. W. (2019). When the muses strike: Creative ideas of physicists and writers routinely occur during mind wandering. Psychological Science, 30(3), 396–404.
Garrison, K. A., Zeffiro, T. A., Scheinost, D., Constable, R. T., & Brewer, J. A. (2015). Meditation leads to reduced default mode network activity beyond an active task. Cognitive, Affective, & Behavioral Neuroscience, 15(3), 712–720.
Goyal, M., Singh, S., Sibinga, E. M., Gould, N. F., Rowland-Seymour, A., Sharma, R., Berger, Z., Sleicher, D., Maron, D. D., & Shihab, H. M. (2014). Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Internal Medicine, 174(3), 357–368.
Heartmath. (2016). EmWave Pro Plus. In https://store.heartmath.com/emwave-pro-plus/
Hoffmann, F., Banzhaf, C., Kanske, P., Bermpohl, F., & Singer, T. (2016). Where the depressed mind wanders: Self-generated thought patterns as assessed through experience sampling as a state marker of depression. Journal of Affective Disorders, 198, 127–134.
Jackowski, A. P., Almeida, A. G., & d., Araújo, C. M. d., Reis, M., Nery, F., Batista, I. R., Silva, I., & Lacerda, A. L. (2012). The involvement of the orbitofrontal cortex in psychiatric disorders: An update of neuroimaging findings. Brazilian Journal of Psychiatry, 34, 207–212.
Khoury, B., Knäuper, B., Schlosser, M., Carrière, K., & Chiesa, A. (2017). Effectiveness of traditional meditation retreats: A systematic review and meta-analysis. Journal of Psychosomatic Research, 92, 16–25.
Killingsworth, M. A., & Gilbert, D. T. (2010). A wandering mind is an unhappy mind. Science, 330(6006), 932–932.
Koelsch, S., Andrews-Hanna, J. R., & Skouras, S. (2022). Tormenting thoughts: The posterior cingulate sulcus of the default mode network regulates valence of thoughts and activity in the brain’s pain network during music listening. Human Brain Map**, 43(2), 773–786.
Konjedi, S., & Maleeh, R. (2017). A closer look at the relationship between the default network, mind wandering, negative mood, and depression. Cognitive, Affective, & Behavioral Neuroscience, 17(4), 697–711.
Kundu, P., Brenowitz, N. D., Voon, V., Worbe, Y., Vértes, P. E., Inati, S. J., Saad, Z. S., Bandettini, P. A., & Bullmore, E. T. (2013). Integrated strategy for improving functional connectivity map** using multiecho fMRI. Proceedings of the National Academy of Sciences, 110(40), 16187–16192.
Kundu, P., Inati, S. J., Evans, J. W., Luh, W.-M., & Bandettini, P. A. (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage, 60(3), 1759–1770.
Lehrer, P., Kaur, K., Sharma, A., Shah, K., Huseby, R., Bhavsar, J., Sgobba, P., & Zhang, Y. (2020). Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta analysis. Applied Psychophysiology and Biofeedback, 45(3), 109–129.
Lehrer, P., Sasaki, Y., & Saito, Y. (1999). Zazen and cardiac variability. Psychosomatic Medicine, 61(6), 812–821.
Lehrer, P., Vaschillo, B., Zucker, T., Graves, J., Katsamanis, M., Aviles, M., & Wamboldt, F. (2013). Protocol for heart rate variability biofeedback training. Biofeedback (online), 41(3), 98.
Lehrer, P. M., & Gevirtz, R. (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5, 756.
Mason, M. F., Norton, M. I., Van Horn, J. D., Wegner, D. M., Grafton, S. T., & Macrae, C. N. (2007). Wandering minds: The default network and stimulus-independent thought. Science, 315(5810), 393–395.
Mather, M., & Thayer, J. F. (2018). How heart rate variability affects emotion regulation brain networks. Current Opinion in Behavioral Sciences, 19, 98–104.
McCraty, R., Atkinson, M., Tomasino, D., & Bradley, R. T. (2009). The coherent heart heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral Review: A Transdisciplinary & Transcultural Journal for New Thought, Research, & Praxis, 5(2).
McMillan, R., Kaufman, S. B., & Singer, J. L. (2013). Ode to positive constructive daydreaming. Frontiers in Psychology, 4, 626.
Milad, M. R., & Rauch, S. L. (2007). The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences, 1121(1), 546–561.
Mrazek, M. D., Franklin, M. S., Phillips, D. T., Baird, B., & Schooler, J. W. (2013). Mindfulness training improves working memory capacity and GRE performance while reducing mind wandering. Psychological Science, 24(5), 776–781.
Nashiro, K., Min, J., Yoo, H. J., Cho, C., Bachman, S. L., Dutt, S., Thayer, J. F., Lehrer, P., Feng, T., Mercer, N., Nasseri, P., Wang, D., Chang, C., Marmarelis, V. Z., Narayanan, S., Nation, D. A., & Mather, M. (2021). Increasing coordination and responsivity of emotion-related brain regions with a heart rate variability biofeedback randomized trial. medRxiv.
O’Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J., & Andrews, C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4(1), 95–102.
Ottaviani, C., & Couyoumdjian, A. (2013). Pros and cons of a wandering mind: A prospective study. Frontiers in Psychology, 4, 524.
Peng, C.-K., Henry, I. C., Mietus, J. E., Hausdorff, J. M., Khalsa, G., Benson, H., & Goldberger, A. L. (2004). Heart rate dynamics during three forms of meditation. International Journal of Cardiology, 95(1), 19–27.
Peng, C.-K., Mietus, J. E., Liu, Y., Khalsa, G., Douglas, P. S., Benson, H., & Goldberger, A. L. (1999). Exaggerated heart rate oscillations during two meditation techniques. International Journal of Cardiology, 70(2), 101–107.
Phongsuphap, S., Pongsupap, Y., Chandanamattha, P., & Lursinsap, C. (2008). Changes in heart rate variability during concentration meditation. International Journal of Cardiology, 130(3), 481–484.
Pizzoli, S. F., Marzorati, C., Gatti, D., Monzani, D., Mazzocco, K., & Pravettoni, G. (2021). A meta-analysis on heart rate variability biofeedback and depressive symptoms. Scientific Reports, 11(1), 1–10.
Poerio, G. L., Totterdell, P., & Miles, E. (2013). Mind-wandering and negative mood: Does one thing really lead to another? Consciousness and Cognition, 22(4), 1412–1421.
Rempel-Clower, N. L. (2007). Role of orbitofrontal cortex connections in emotion. Annals of the New York Academy of Sciences, 1121(1), 72–86.
Rodriguez-Larios, J., de Oca, E. A. B. M., & Alaerts, K. (2021). The EEG spectral properties of meditation and mind wandering differ between experienced meditators and novices. Neuroimage, 118669.
Rolls, E. T. (2004). The functions of the orbitofrontal cortex. Brain and Cognition, 55(1), 11–29.
Rolls, E. T. (2019). The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia, 128, 14–43.
Rolls, E. T., Cheng, W., & Feng, J. (2020). The orbitofrontal cortex: reward, emotion and depression. Brain Communications, 2(2), fcaa196.
Sarmiento, S., García-Manso, J. M., Martín-González, J. M., Vaamonde, D., Calderón, J., & Da Silva-Grigoletto, M. E. (2013). Heart rate variability during high-intensity exercise. Journal of Systems Science and Complexity, 26(1), 104–116.
Seli, P., Carriere, J. S., & Smilek, D. (2015). Not all mind wandering is created equal: Dissociating deliberate from spontaneous mind wandering. Psychological Research Psychologische Forschung, 79(5), 750–758.
Seli, P., Kane, M. J., Smallwood, J., Schacter, D. L., Maillet, D., Schooler, J. W., & Smilek, D. (2018). Mind-wandering as a natural kind: A family-resemblances view. Trends in Cognitive Sciences, 22(6), 479–490.
Smallwood, J., & Schooler, J. W. (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66, 487–518.
Tarvainen, M. P., Niskanen, J.-P., Lipponen, J. A., Ranta-Aho, P. O., & Karjalainen, P. A. (2014). Kubios HRV–heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220.
Thayer, J. F., & Lane, R. D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216.
Thayer, J. F., & Lane, R. D. (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 33(2), 81–88.
Thomas Yeo, B., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., Roffman, J. L., Smoller, J. W., Zöllei, L., & Polimeni, J. R. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165.
Thorsen, A. L., Hagland, P., Radua, J., Mataix-Cols, D., Kvale, G., Hansen, B., & van den Heuvel, O. A. (2018). Emotional processing in obsessive-compulsive disorder: A systematic review and meta-analysis of 25 functional neuroimaging studies. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 563–571.
van Agteren, J., Iasiello, M., Lo, L., Bartholomaeus, J., Kopsaftis, Z., Carey, M., & Kyrios, M. (2021). A systematic review and meta-analysis of psychological interventions to improve mental wellbeing. Nature Human Behaviour, 1–22.
Weng, H. Y., Lewis-Peacock, J. A., Hecht, F. M., Uncapher, M. R., Ziegler, D. A., Farb, N. A., Goldman, V., Skinner, S., Duncan, L. G., & Chao, M. T. (2020). Focus on the breath: Brain decoding reveals internal states of attention during meditation. Frontiers in Human Neuroscience, 336.
Williams, D. P., Cash, C., Rankin, C., Bernardi, A., Koenig, J., & Thayer, J. F. (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in psychology, 6.
Zhou, H.-X., Chen, X., Shen, Y.-Q., Li, L., Chen, N.-X., Zhu, Z.-C., Castellanos, F. X., & Yan, C.-G. (2020). Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage, 206, 116287.
Zuckerman, M., Hodgins, H. S., Zuckerman, A., & Rosenthal, R. (1993). Contemporary issues in the analysis of data: A survey of 551 psychologists. Psychological Science, 4(1), 49–53.
Acknowledgements
The authors thank our research assistants for their help with data collection: Michelle Wong, Kathryn Cassutt, Collin Amano, Paul Choi, Heekyung Rachael Kim, Seungyeon Lee, Alexandra Haydinger, Lauren Thompson, Gabriel Shih, Divya Suri, Sophia Ling, Akanksha Jain, and Linette Bagtas.
Funding
This study was supported by NIH R01AG057184 (PI Mather).
Author information
Authors and Affiliations
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nashiro, K., Yoo, H.J., Min, J. et al. Effects of a randomised trial of 5-week heart rate variability biofeedback intervention on mind wandering and associated brain function. Cogn Affect Behav Neurosci 22, 1349–1357 (2022). https://doi.org/10.3758/s13415-022-01019-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-022-01019-7