Abstract
Background
Pancreatoduodenectomy (PD) has a considerable surgical risk for complications and late metabolic morbidity. Parenchyma-sparing resection of benign tumors has the potential to cure patients associated with reduced procedure-related short- and long-term complications.
Materials and Methods
Pubmed, Embase, and Cochrane libraries were searched for studies reporting surgery-related complications following PD and duodenum-preserving total (DPPHRt) or partial (DPPHRp) pancreatic head resection for benign tumors. A total of 38 cohort studies that included data from 1262 patients were analyzed. In total, 729 patients underwent DPPHR and 533 PD.
Results
Concordance between preoperative diagnosis of benign tumors and final histopathology was 90.57% for DPPHR. Cystic and neuroendocrine neoplasms (PNETs) and periampullary tumors (PATs) were observed in 497, 89, and 31 patients, respectively. In total, 34 of 161 (21.1%) patients with intraepithelial papillar mucinous neoplasm exhibited severe dysplasia in the final histopathology. The meta-analysis, when comparing DPPHRt and PD, revealed in-hospital mortality of 1/362 (0.26%) and 8/547 (1.46%) patients, respectively [OR 0.48 (95% CI 0.15–1.58); p = 0.21], and frequency of reoperation of 3.26 % and 6.75%, respectively [OR 0.52 (95% CI 0.28–0.96); p = 0.04]. After a follow-up of 45.8 ± 26.6 months, 14/340 patients with intraductal papillary mucinous neoplasms/mucinous cystic neoplasms (IPMN/MCN, 4.11%) and 2/89 patients with PNET (2.24%) exhibited tumor recurrence. Local recurrence at the resection margin and reoccurrence of tumor growth in the remnant pancreas was comparable after DPPHR or PD [OR 0.94 (95% CI 0.178–5.34); p = 0.96].
Conclusions
DPPHR for benign, premalignant neoplasms provides a cure for patients with low risk of tumor recurrence and significantly fewer early surgery-related complications compared with PD. DPPHR has the potential to replace PD for benign, premalignant cystic and neuroendocrine neoplasms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic cystic neoplasms represent an increasingly detected entity of tumors of 10–15% of all pancreatic cystic lesions.1 Intraductal papillary mucinous neoplasms (IPMNs), mucinous cystic neoplasms (MCNs), and solid pseudopapillary neoplasms (SPNs) are primarily benign tumors but have variable inherent risks of malignancy. Serous cystic neoplasms (SCNs) are considered to be of benign nature. The risk for malignant transformation ranges up to 60%2 for main-duct (MD) or mixed-type IPMN, 6–46%3 for branch-duct (BD) IPMN, and up to 15%4 for MCN. IPMNs are predominantly located in the pancreatic head, whereas MCNs are detected more frequently in the body and tail. SPN are classified as low-grade malignant neoplasms, although 12–18% are malignant tumors in the final histopathology after surgical treatment.5
Pancreatic neuroendocrine neoplasms (PNETs) account for approximately 2% of all pancreatic tumors.6 They are heterogeneous neoplasms with a variable malignant potential for non-functional and functional neoplasms. Approximately 30–40% of pancreatic neuroendocrine neoplasms are located in the pancreatic head and neck.7 Goals of surgical treatment of benign and premalignant cystic neoplasms are cure of the patient, relief from symptoms by resection, prevention of development of a malignant pancreatic tumor, low risk for surgery-associated complications, and maintenance of pancreatic and upper-gastrointestinal tract (GI tract) tissues and functions.
The high level of surgical techniques and standardization, high quality of intensive care unit management, use of nonoperative interventions for complications, and surgical expertise in many centers have led to the consideration of Whipple resection or pylorus-preserving pancreaticoduodenectomy (PPPD) with increasing acceptance as the appropriate surgical treatment for benign tumors and premalignant cystic neoplasms (CNs) of the pancreatic head.8,9 However, the use of PD for patients suffering benign, premalignant, cystic, or neuroendocrine neoplasms questions the classical surgical treatment with respect to multiorgan tissue loss for a benign, local pancreatic disease.10 Despite a decrease in reported mortality and local complications, PD remains a complex, multiorgan resection with distinct surgery-associated complications, considerable mortality, and late metabolic morbidity.10,11,12,13,14,15,16 The development and increasing use of parenchyma-sparing, local resection of benign pancreatic tumors—tumor enucleation (TE),17 duodenum-preserving pancreatic head resection (DPPHR)18 and pancreatic middle segment resection19—parallels the increase in the number of patients with symptomatic or accidentally detected, asymptomatic, benign neoplasms requiring surgical treatment. The rationale for local resection of benign tumors of the pancreatic head are (i) cure of patients; (ii) preservation of the duodenum, extrahepatic biliary ducts, gastric antrum, and pylorus; (iii) maintenance of the functional integrity of the duodenum regarding coordination of digestive metabolic and motility functions; and (iv) maximum conservation of the pancreatic tissue. DPPHR for benign pancreatic head tumors has the advantage of conservation of the duodenum and first jejunal loop and a limited loss of pancreatic and biliary tissues. New onset of diabetes mellitus (DM) and new onset of pancreatic exocrine insufficiency (PEI) are assessed to be low following DPPHR;18 in most patients, endocrine and exocrine functions were measured as being at the preoperative level.20,21,22 Many institutions reported low rates of surgery-associated complications regarding frequencies of reoperation, reintervention, and in-hospital mortality after DPPHR.20 Maintenance of endocrine and exocrine pancreatic and upper-GI tract functions are documented by studies with high clinical evidence.21,22 However, data regarding oncologic outcome following DPPHR are lacking. Consequently, this systematic review and meta-analysis aims to evaluate the pattern of long-term outcomes regarding tumor recurrence and late mortality comparing DPPHR and PD. The hypothesis was that DPPHR applied for benign tumors ensures the cure of patients, and is, compared with data following PD, associated with a low risk for procedure-related surgical morbidity. The primary endpoints were the metrics for postoperative complications and for oncologic outcome, frequency and type of tumor recurrence, anastomotic tumor recurrence, and tumor reoccurrence in the remnant pancreas based on the final histopathology after DPPHR or PD.
Materials and Methods
Search Strategy
We conducted a comprehensive literature search of the PubMed/Medline, Embase, and Cochrane databases. For PubMed, a search for medical subject heading (MeSH) terms was applied. For Embase and Cochrane, searches with Emtree and MeSH terms were performed, respectively, including a text word search for surgical techniques. A text word search for pancreatic resection techniques including duodenum-sparing head resection and pancreatoduodenectomy for benign tumors was performed. The following search items were used: duodenum-preserving pancreatic head resection, parenchyma-sparing surgery for pancreatic head tumors, pancreatoduodenectomy for benign tumors, Whipple resection for cystic neoplasms, Whipple resection for neuroendocrine tumors, pancreatic head resection with segment resection of the duodenum, local resection of periampullary tumors, severe dysplasia, and advanced cancer in resected benign pancreatic head tumors.
Studies reporting limited surgery for cystic neoplasms, neuroendocrine tumors of the pancreatic head, or low-risk periampullary tumors were included in the selection process. The preoperative diagnosis and final histological diagnostic pattern of benign tumors of the pancreatic head included IPMN, MCN, SPN, serous cystic adenoma (SCA), non-functional and functional PNETs, periampullary tumors, inflammatory tumors of chronic pancreatitis, and other tumors. The search results for identification of relevant publications are presented in Fig. 1. Case reports, case series up to four patients, reports of assessment of metabolic functions after pancreatic head resection, and studies including advanced pancreatic head tumors in preoperative diagnosis were excluded. Figure 1 shows the PRISMA flow diagram of the selection process.23 The publications were checked for cross references that were eligible as additional reports that were not identified by the primary search items. Differences were resolved by mutual agreement between two authors (H.G.B. and B.P.).
Evaluation of Methodological Quality of Studies
The methodological quality of the 38 studies finally included in the systematic review and of the 14 studies included in the meta-analysis was assessed using the Critical Appraisal Skills Program of the Oxford Centre for Evidence-Based Medicine.24 The manuscripts were evaluated according to this program for the level of evidence; specifically, criteria for selection bias, measure bias, and applicability were assessed for each study. Additionally, the Newcastle-Ottawa Scale (NOS) was applied to assess the quality of the controlled, prospective, and retrospective cohort studies, ensuring an objective evaluation of the most basic quality aspects of non-randomized cohort studies with regard to selection criteria, case definition, representativeness of cases, application of international histopathological criteria, selection of controls, comparability of study groups, and assessment of outcome variables.25 Cohort studies with scores of 8 or 9 were considered to have good-to-high levels of evidence and were included in the analysis (Tables 1 and 2).
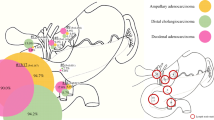
Duodenum-Preserving, Total, or Partial Pancreatic Head Resection
DPPHRt involves resection of the pancreatic head while conserving the pancreatic neck, intrapancreatic common bile duct (CBD), and duodenum (Supplemental Material, Fig. S2A). A subgroup of DPPHRt comprises patients who underwent resection of the peripapillary segment of the duodenum (DPPHRt+sd) and resection of the intrapancreatic CBD (Supplemental Material, Fig. S2B). A few patients who underwent near total pancreatic head resection by conserving some suprapapillary pancreatic tissue of the groove of the pancreas are included in the DPPHRt group. Partial pancreatic head resection (DPPHRp) was performed when tumor size and the proposed biological nature of the neoplasm necessitated tissue resection extending beyond the pancreatic main duct. DPPHRp does not require resection of the duodenum and/or CBD; the tissue outside of the tumor wall of the ventral or dorsal pancreatic head is preserved (Supplemental Material, Fig. S2C). Reconstruction techniques were predominantly pancreatico-jejunostomy, pancreatico-gastrostomy, and pancreatico-duodenostomy.
Data Extraction Process
The presented data are based on a selective evaluation of 38 studies dealing with DPPHR published between 1994 and 2022. Data extraction from each study was conducted independently by two authors (H.G.B. and B.P.) according to the lists of prespecified selection criteria. To evaluate the intraoperative and early postoperative outcomes, the following criteria were used for analysis: in-hospital mortality, reoperation, and tumor size.
The final histology of the tumors was listed separately, including IPMN, MCN, SPN, SCA, and pancreatic non-functional and functional PNETs, as well as periampullary tumors originating from the peripapillary duodenum, papilla or ampulla, and prepapillary CBD. Chronic pancreatitis and other cysts and tumors were additionally listed. Advanced pancreatic cancer, preoperatively considered to be benign tumor, but identified by frozen section investigation intraoperatively and/or by final histopathological diagnosis, was listed separately in the respective group of neoplasm: IPMN, MCN, and PNET and PAT. For IPMN and MCN, specific criteria with respect to MD- or BD-IPMN and the degrees of dysplasia and minimal invasive or focal carcinoma were listed separately and included in the analysis when clear definitions were reported in the manuscript. High-grade dysplasia of IPMN and MCN were listed as benign, avoiding the term carcinoma in situ.26 Regardless of the staging of the reports (minimally invasive, focal, or micro-carcinoma or T1–2 carcinoma), we used the term “cancer arising in association with IPMN” (“IPMC”) or “invasive cancer” for cancer. With respect to long-term outcome after DPPHR or PD for benign tumors, the type and frequency of tumor recurrence were listed. Specifically, a recurrent tumor at the resection margin, metachronous reoccurrence of tumor in the remnant pancreas, and extrapancreatic metastazation were documented separately as tumor recurrence. All patients with advanced cancer identified by frozen section or/and final histopathologic assessment experienced either a conversion to PD intraoperatively or early postoperative re-surgery by PD and/or DPPHR plus adjuvant chemotherapy during the index hospitalization; these patients were kept in the follow-up registry of the cohorts.
The indication for DPPHR or PD was based on the presence of abdominal symptoms in approximately 85% of patients. All tumors were considered preoperatively to be of benign nature, except some patients with papillary/ampullary tumors (PATs). Periampullary tumors were subdivided into tumors derived from the peripapillary duodenum, adenomas of the papilla/ampulla, or tumors of the peripapillary CBD, including pancreaticobiliary maljunction. Evaluating long-term outcomes, data regarding time, and reason for late mortality during the reported follow-up period were separately listed. Seven authors were contacted to clarify the cause and type of postoperative interventions and histological classification of the tumor that were lacking in their respective publications.43,45,55,56,58,60,63 The reported period covers 27 years. The criteria grade of dysplasia, BD-, MD-, and mixed-type IPMN, and type of recurrent tumor are incompletely reported because the clinical histopathological criteria were only established as guideline metrics in recent years.
Statistical Analysis
All analyses were conducted using R for statistical computing version 4.2.2 (www.r-project.org, package meta). Continuous variables were expressed as mean ± standard deviation (SD), whereas categorical variables were presented as absolute frequencies and percentages. Explorative statistical testing of the DPPHR subgroups (total versus partial resection) was performed using the chi-squared test. Statistical significance was set at p < 0.05. For the meta-analytic approach, odds ratio (OR, Mantel–Haenszel method) was used for all considered dichotomous outcomes.27 All effect estimates were presented together with their 95% confidence intervals (CIs). To assess the extent of between-study heterogeneity, the I2 statistic was evaluated, leading to the application of a fixed-effects model where I2 was < 40%; otherwise, a random effects model was used. A graphical representation of the results was based on forest plots. To determine whether significant publication bias had to be assumed, funnel plots were additionally created.
Results
Study Groups
The analysis was based on 38 good- to high-quality cohort studies, presenting data from 729 patients following DPPHR (Tables 1 and 2). A total of 533 patients included in the meta-analysis underwent PD for benign tumors, premalignant neoplasms, or low-risk malignant periampullary tumors. The systematic review was performed by analyzing the DPPHR-related data of all patients of the 38 cohort studies. DPPHRt was performed in 499 patients and DPPHRp in 230 patients. The meta-analysis was based on data from 14 controlled studies, including a control group of patients who underwent PD. In the meta-analysis, the results of 367 patients following DPPHR were compared with 533 patients following PD, of whom 151 underwent Whipple resection and 382 PPPD.
Assessment of Methodological Quality of Studies
The systematic review was based on 24 cohort studies in the review group (Table 1) and on 14 studies in the meta-analysis group (Table 2). In total, 21 studies were controlled cohort studies, of which 14 were prospective and seven retrospective reports. Seventeen reports were without a control group, of which 12 were prospective studies. The critical appraisal for methodology revealed 26 studies with evidence level 2 and 12 studies with evidence level 3. Evidence level 2 certifies a good-quality cohort study. Additionally, NOS score was applied to assess the quality of all cohort studies, which enabled an objective evaluation of the most basic quality aspects of nonrandomized studies. In total, 30 cohort studies elicited a score of ≥ 8; mean NOS score was 8.1, which indicated a good quality of the cohort studies.
Results of Baseline Data
The baseline data of the 38 cohort studies, composed of 1262 patients, are presented in Tables 1 and 2. These studies included data from 729 patients following DPPHR and 533 patients following PD for benign tumors. In total, 27 studies were published between 2010 and 2022. The concordance of preoperative diagnosis of benign tumors and the final histopathology after DPPHR was 90.57%. Patients who experienced DPPHRp for chronic pancreatitis were excluded from the calculation of concordance. In the review group (Table 1), the mean age of the patients was 49.7 (SD ± 12.6) years and in the meta-analysis group, the mean age was 49.9 (SD ± 12.5) years (Table 2). The gender relationship M:F was 1:1.75 in the review group and 1:1.04 in the meta-analysis group. Two studies reported results after the application of DPPHR in adolescents and children, predominantly for SPN.38,57
Results of Tailored Use of Duodenum-Preserving Pancreatic Head Resection
In total, 499 patients (68.4%) underwent DPPHRt and 230 (31.6%) DPPHRp (Table 3). Tumor size of the DPPHRt group was significantly larger than in the DPPHRp group (3.7 cm versus 2.9 cm, respectively, p = 0.001). In the DPPHRt group, 367 patients experienced complete preservation of the duodenum, and 173 patients underwent resection of the peripapillary segment of the duodenum and the CBD (DPPHRt+sd). In 189 patients who underwent DPPHRp, the duodenum and the intrapancreatic CBD were preserved, except in 7 patients, who underwent additional CBD resection.
In-hospital mortality was 0% after DPPHRp. Following DPPHRt, 1/382 (0.26%) patients died (Supplemental Material, Fig. S2A). The frequency of reoperation was significantly lower following DPPHRt, with 3.3% versus 6.8% following PD [OR 0.52 (95% CI 0.26–1.04); p = 0.04].
GI tract reconstruction was performed with end-to-end anastomosis of the duodenum in 173 patients; in 199 patients an anastomosis of the CBD with the duodenum was performed. The GI tract reconstruction with the left pancreas was carried out with an excluded jejunal loop in 441 patients, with the stomach in 206 patients, with the duodenum in 67 patients, and as a duct-to-duct anastomosis of the pancreatic main duct (PMD) in 15 patients. In ten patients, duodenum-preserving total pancreatectomy was carried out with conservation of the spleen.
Of the 533 patients who underwent PD for benign tumors of the pancreatic head, Whipple resection was performed in 132 and PPPD in 401 (Table 2). When undergoing PD for benign tumors of the pancreatic head, in most patients pancreaticojejunostomosis was performed, in 104 patients a pancreaticogastrostomosis was performed, and in 181 patients a laparoscopic or robotic-assisted DPPHR was performed.
The final histopathologic diagnosis revealed 472 patients with benign CNs. Of these patients, advanced cancer was revealed in 25, including IPMN-associated minimally invasive, micro-, or focal cancer in 9; T1/2 in 14; and MCC and PDAC in 1 each. A total of 78 patients displayed a benign PNET in the final histopathology, of whom 55 presented a non-functional, benign neoplasm. A total of 11 patients from the PNET group displayed a malignant tumor: malignant gastrinoma 1 patient, carcinoid of the papilla in 4 patients, carcinoid of the duodenum in 1 patient, and islet cell carcinoma in 5 patients. A total of 31 patients displayed tumors of the papilla/ampulla or peripapillary CBD and/or maljunction of the pancreatic and biliary ducts. Other tumors were reported for 112 patients (Table 3), with chronic pancreatitis in 95 of these patients. Under ‘other tumors', patients were operated on with the diagnosis of benign neoplasm, and three patients presented histopathologically a metastasis of renal cell carcinoma (two patients) or metastasis of an ileum carcinoid (one patient). Of 18 patients who displayed advanced cancer intraoperatively or by final histological assessment of the operative specimen, 6 patients had a conversion to classical Whipple OP, 2 patients had re-surgery PD during the index hospitalization, and 10 patients experienced an additional adjuvant chemotherapy period.
Results of Histopathological Assessment of the Operative Specimen following DPPHR
In total, 258 patients revealed IPMN and 57 patients MCN (Table 3). The IPMN differentiation in MD- and BD-duct and mixed-type IPMN was reported for 137 patients. Of them, 111 underwent surgery for BD-IPMN (81.0%). Predominantly DPPHRt or DPPHRt plus segment resection of the peripapillary duodenum was performed for MD- or BD-IPMN. With respect to the degree of dysplasia, 34 of 161 patients with IPMN (21.1%) were assessed to have severe dysplasia, whereas for MCN only 1 of 57 patients displayed severe dysplasia (Table 4). Advanced cancer in IPMN or MCN was found in 25 patients. In 13 of the 25 patients who exhibited advanced cancer intraoperatively by frozen section or by final histopathologic assessment of the operative specimen, 6 patients underwent a conversion to classical Whipple procedure and 2 patients underwent re-surgery during the index hospitalization applying a radical PD. Five patients received additional adjuvant chemotherapy after total DPPHR.
The final histopathology of 89 patients with PNETs following DPPHR is presented in Table 5. In total, 55 patients experienced DPPHRt and 23 DPPHRp. Of 78 patients with PNET, 55 were classified as non-functional neoplasm. The tumor diameter for this subgroup of patients is infrequently reported, but was documented in six reports to be 1.4–2.5 cm. Of 23 patients with PNET, sporadic insulinoma was observed in 16. In the subgroup of functional PNETs, malignant tumors were found in 11 patients (Table 5).
In the subgroup of 31 patients with PATs, advanced cancer was observed in 12 patients (38.7%): most frequently T1 cancer and T1a carcinoma in adenoma of the papilla in 4 patients; T1 carcinoma of the ampulla in 1 patient; T1 of the peripapillary CBD in 3 patients; and CBD cancer T2 in 1 patient. Almost all patients with periampullary tumor experienced DPPHRt+sd (Supplemental Material, Fig. S2C) (Table 5).
Concordance of Diagnosis of Benign Tumor
The concordance of preoperative and postoperative diagnosis of “benign tumor” based on the final histopathology was 90.57%. In patients with CNs, PNETs, or PATs, advanced malignoma was finally found in 7.05%, 12.04%, and 38.7%, respectively. A total of 18 patients who presented intraoperative signs of advanced cancer were considered for DPPHR for benign tumor and underwent intraoperative conversion to PD or re-surgery during the index hospitalization, applying a radical PD or receiving additional adjuvant chemotherapy.
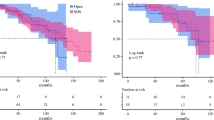
Results of Meta-analysis Comparing DPPHRt and PD for Frequency and Type of Recurrent Tumor
The meta-analysis was based on 14 studies published between 2010 and 2022 comparing the histopathological data following DPPHRt or PD for benign tumor. Local anastomotic recurrence was observed in 2 of 230 patients following DPPHRt (0.86%) and in 5 of 294 patients following PD (1.7%) (Supplemental Material, Fig. S2C). The forest plots applying overall odds ratio fixed effects and random effects model show heterogeneity and a comparable frequency of recurrence at the resection margin after either type of resection (Supplemental Material, Fig. S2C) (p = 0.95). Following DPPHR, tumor reoccurrence in the remnant pancreas was observed in 3 of 206 patients (1.5%) and in 5 of 224 patients with PD (2.2%). The test for overall effect showed no difference between DPPHRt and PD (Fig. 2D) (p = 0.73). Regarding the type and frequency of tumor recurrence after DPPHRt in both study groups (review and meta-analysis group), 9 of 257 patients experienced tumor recurrence or reoccurrence (3.5%), respectively, in the long-term follow-up of 45.8 ± 26.6 months (Table 4). Three patients displayed IPMN recurrence at the resection margin, four patients developed reoccurrence of the tumor in the remnant pancreas, and two patients developed extrapancreatic metastases after local resection of IPMN-associated cancer. After DPPHRp, three patients developed reoccurrence of IPMN in the remnant pancreas, two of them with primarily benign IPMN (Fig. 3), one patient developed cancer of MCN at the resection margin, and one patient peritoneal metastization after intraoperative dissemination of an IPMC. (Table 4). Recurrent tumor following DPPHRt for PNET was observed in 2 of 62 patients, with one each succumbing to metastases of a gastrinoma or islet cell carcinoma (Table 5). In addition, 1 of 31 patients developed multiorgan metastases of an advanced carcinoma of the papilla/ampulla after DPPHRt (Table 5).
Comparing baseline data of the DPPHR (367 patients) and PD subgroups (533 patients) included in the meta-analysis, the following criteria displayed similar results: male/female gender and frequencies of CNs (62.7% versus 58.9%), PATs (8.4% versus 7.1%), other tumors (4.6% versus 2.8%), and cancer/malignoma (7.9% versus 7.8%). In the DPPHR group, the frequencies of PNETs (24.3 versus 13.5%; p < 0.001) and CP (25.9% versus 18.2%; p < 0.006) were higher than in the PD group. The PD group displayed a significantly higher mean age (52.5 versus 47.9 years; p < 0.001) and a larger tumor size (4.1 versus 3.5 cm; p < 0.001). In the DPPHR group, for 153 of 367 patients a duct origin of the IPMN was reported. Approximately two-thirds of patients with IPMN who underwent DPPHR displayed a BD-type, whereas in the PD group, the MD- and mixed-type IPMN prevailed in 184 of 533 patients wtih IPMN.
Discussion
The review and meta-analysis include 729 patients who underwent duodenum-preserving pancreatic head resection for benign tumors. The analysis presents the first cumulative data of long-term outcome of 634 patients after undergoing local, parenchyma-sparing pancreatic head resection for cystic neoplasms, neuroendocrine tumors, or periampullary neoplasms.
After a mean follow-up of 45.8 months, the risk of development of a tumor recurrence after DPPHR at the resection margin was 1.21%. The reoccurrence of a benign neoplasm or an adenocarcinoma in the remnant pancreas was observed in seven patients (2.1%). The data underline that local extirpation of a benign or potentially malignant neoplasm applying DPPHR was associated with low risk of tumor recurrence. Comparing DPPHR with PD, the frequency of local tumor recurrence revealed that both surgical techniques have similarly high levels of long-term patient cure. Additionally, DPPHR has the advantages of low in-hospital mortality and a significantly lower frequency of reoperation and reinterventions compared with PD. With regard to long-term patient cure, a low risk of tumor recurrence, significantly reduced surgery-associated complications,65 and maintenance of metabolic pancreatic and upper-GI tract functions,15 DPPHR performance is to be weighed against the risk of carrying out a PD.
Predicting the risk of malignancy, international guidelines established criteria for CNs for decision-making as defined by an absolute indication for surgery, surveillance of patients for IPMN and MCN, and definition of worrisome features and high-risk stigmata.66,67,68,69
Diagnostic tools, including high-resolution magnetic resonance tomography (MRT), liquid biopsy, and fine needle aspiration, have led to surgeries being less frequently performed too late in the case of invasive cancer, or too early in patients with a distinct risk of malignant transformation.70,71,72
Applying parenchyma-sparing pancreatic head resection for CNs lowers the surgical risk and postoperative morbidity, even though too early or too late surgical treatment equally applies for the parenchyma-sparing surgical techniques. Most patients included in this review and meta-analysis underwent surgical treatment due to clinical symptoms.
Decision-making for surgery in asymptomatic patients under surveillance for IPMNs, MCNs, or SPNs should occur before the development of an invasive carcinoma. However, radiological factors, clinical signs, tumor markers, and even DNA analysis of the IPMN are presently not sufficiently accurate to predict the risk or the presence of malignant tumor with high sensitivity or specificity.70,71,72 Resection of suspected asymptomatic IPMN lesions through a duodenum-sparing resection is associated with low postoperative risk for complications and maintenance of metabolic and upper-GI tract functions and is thereby the basis for a cancer-preventive surgical treatment.72,73
In a recently published analysis of a large IPMN cohort of 1074 patients including benign and advanced neoplasms, recurrence rate was 14.4% at a median of 24 months.74 Recurrence was defined as radiographic or histologic diagnosis of a metachronous tumor in the remnant pancreas or appearance of a tumor outside the pancreas.74 Recurrence in this analysis included tumor growth at the resection margin, metachronous tumor reoccurrence in the remnant pancreas, or extrapancreatic, metastatic tumor growth in the follow-up time. The recurrence rates following DPPHR among 258 patients with benign, noninvasive IPMN, including the 24 patients with cancer arising in association with IPMN, was 5.03% after a mean follow-up of 45.8 months. Four patients developed tumor recurrence located at the resection margin tissue, six patients experienced reoccurrence of an IPMN or a PDAC in the remnant pancreas, and three patients developed extrapancreatic metastization. Frozen section control of the resection margin is recommended in any case of local tumor extirpation. For cystic neoplasms of the pancreatic head, tumor size or tumor location close to the duodenal wall and/or compression of the intrapancreatic CBD are criteria for selection of the type of DPPHR. DPPHRt (type II) was used for BD- and mixed-type IPMN and for MCN. DPPHRt+sd (type III) including segment resection of the peripapillary duodenum and the intrapancreatic CBD was applied for MD-IPMN of the pancreatic head. DPPHRp for SCN and SPN was associated with the advantage of preserving the intrapancreatic CBD and minimizing tissue loss of the pancreatic head. Performing total or partial DPPHR for SPN is considered a major advantage and as a cancer-preventive treatment, because most patients at the child and adolescent age are female.34,55
During the long-term outcome, seven patients developed metachronous tumor appearance in the remnant pancreas, clearly as a consequence of a multifocal disease. This may be attributed to the difference in the fundamental biology of the disease driving the process of malignant transformation.75,76 These data underline that a postoperative surveillance protocol should be maintained after local, parenchyma-sparing resection of the pancreatic head for patients with the risk for multifocality of the neoplasm. The development of extrapancreatic cancer recurrence after surgical treatment of IPMN was equally low following PD and DPPHR. Interestingly, 9 of 25 patients, who were classified in the final histopathology as micro-carcinoma or focal cancer of IPMN, did not develop local or systemic recurrence in the mean follow-up time of 45 months.
Habib et al. found that the pattern and timing of recurrence after resection of an invasive carcinoma arising in association with IPMN was significantly longer for recurrence at resection margin (21.6 months) than for systemic recurrence (11.4 months).26 Independent predictors of systemic recurrence are R1 margin positivity for high-grade dysplasia (HGD) or invasive cancer and poor differentiation. Regarding the histologic pattern of IPMNs, data from Koh et al. demonstrated that the pancreatobiliary subtype was associated with a higher risk for recurrence, whereas the gastric subtype was found to have a lower risk for recurrence.77 Systemic cancer recurrence was observed after DPPHR of advanced cancer originating from cystic neoplasm in three patients. Distant metastization after resection of cystic neoplasm predominantly develop in up to three postoperative years, which was covered by the follow-up time.
In this review, 21.1% of patients with IPMN, whose pattern of dysplasia of the neoplasm was reported, exhibited severe dysplasia in the final histopathology. None of the patients with severe dysplasia of IPMN were reported to have developed tumor recurrence after DPPHR. Local, parenchyma-sparing pancreatic head resection using DPPHRt (types II or III) for benign IPMN containing severe dysplasia is a safe treatment to cure the patient. On the basis of genomic analysis, data from Noe et al. revealed an average window of more than 3 years between HGD and the development of an invasive pancreatic cancer.78 IPMN with the presence of HGD is considered to be a risk factor for the subsequent development of pancreatic ductal adenocarcinoma.79,80,81,82 Therefore, presence of HGD in IPMN is a criterion for cancellation of surveillance and a change without delay to surgery, applying an adequate parenchyma-sparing surgical treatment.
PNETs
DPPHR was performed in 89 patients with PNETs. The final histopathologic investigation revealed predominantly non-functional neoplasms. Tumor size was 2.97 and 3.7 cm (p ≤ 0.001) after partial or total DPPHR, respectively. Current 2016 guidelines of the European Neuroendocrine Tumor Society (ENET) recommend resection for all functional, sporadic PNETs regardless of tumor size.83 For non-functional PNETs, surveillance is favored for tumors ≤ 2 cm, except for patients with symptoms or higher tumor grade or presence of enlarged lymph nodes.84 For non-functional PNETs of the pancreatic head, a local DPPHRp was preferentially applied and displayed an in-hospital mortality of 0%. For PNETs ≥ 3 cm in size or the presence of sporadic insulinoma, duodenum- and CBD-preserving total head resection was applied. Lymph node allocation is recommended for each patient when undergoing local or classical resection for PNET.
DPPHRt with segment resection of the peripapillary duodenum and intrapancreatic CBD was carried out in five patients with carcinoid tumors, one of the duodenum and four of the papilla. ENET guidelines recommend surveillance for non-functional PNETs ≤ 2 cm if the tumor is grade 1 or low grade 2 and asymptomatic.83 However, recently published results presented data to expand the scope of surgical removal of tumors with a size of 1–2 cm.85,86,87 The increase in the recognition of PNETs has led clinicians to reconsider operative approaches to small, non-functional tumors of 1–2 cm.88 Tumor enucleation is the most favored surgical treatment for small, non-functional PNETs.86,88 However, tumor enucleation for PNETs located in the pancreatic head have a high risk of pancreatic fistula B or C, following opening of one of the pancreatic main ducts. Injuring of the pancreatic main ducts is frequently associated with local complications, increasing risk for re-interventions, and leading to longer hospital stays.89,90 A survival benefit was found for resection of PNETs > 1 to < 2 cm out of 3243 cases of < 2 cm when performing a classical pancreatic head resection or tumor enucleation.88 DPPHR for pancreatic head PNETs including sporadic non-functional and functional PNETs was performed in 89 patients with an in-hospital mortality of 0%. DPPHR for PNETs of the pancreatic head was associated with low levels of POPF B+C, low frequency of re-interventions, and maintenance of endocrine and exocrine pancreatic functions. Histopathologic diagnosis and staging of neuroendocrine tumors of the pancreatic head should also be based on lymph node investigation. In the group of partial DPPHR, lymph node dissection for PNETs was not routinely performed; the staging of the patients with non-functional PNET after partial DPPHR was incomplete.
Periampullary Tumors
The concordance between pre- and postoperative diagnosis of benign periampullary tumor was 61.3%. In the final histopathology, 12 of 31 patients exhibited T1 cancer in association with villous or tubulovillous adenoma. Villous or tubulovillous adenomas are the most common benign lesion, bearing a significant malignant potential.91 In this series of 31 periampullary tumors, 38.7% displayed advanced cancer in the final histopathologic examination, most of them T1 stage cancer. All patients were preoperatively managed by endoscopic surveillance programs including endoscopic resection procedures. The patients were finally referred for surgical treatment. Histopathological assessment of biopsy material revealed carcinoma in small adenomas of the papilla in more than 20% and in large villous adenomas in up to 60%.92,93 Except for 5 patients, who exhibited a benign peripapillary common bile duct lesion, 26 patients had a DPPHRt with segment resection of the peripapillary tumor-bearing segment of the duodenum and resection of the CBD. Of note, final histopathology in five additional patients revealed carcinoid tumor of the papilla (four patients) or the peripapillary duodenum (one patient).These patients are listed in the group of PNETs according to international guidelines (Table 5).The in-hospital mortality was 0% for 31 patients with PAT. DPPHRt with segment resection of the peripapillary duodenum and CBD is recommended for peripapillary and ampullary adenomas, which have histologically severe dysplasia or are associated with T1 carcinoma. Dissection of N1 and N 2 lymph nodes around the pancreatic head was performed in association with DPPHR for tumor staging without extension of the surgical procedure. Local tumor recurrence after DPPHRt+sd was not observed in the long-term follow-up, except for one patient who succumbed to liver metastization following resection of advanced cancer of the papilla. Applying total DPPHR for patients with advanced papillary/ampullary cancer, notably for patients with distal CBD cancer, is an inadequate surgical treatment. Whipple resection is recommended.
Summary
DPPHR was performed in 729 patients for benign tumors of the pancreatic head, premalignant cystic neoplasms, neuroendocrine tumors, or low malignant peripapillary/ampullary adenomas. The concordance of the preoperative diagnosis of benign tumor with the final histopathology was 90.57%. In-hospital mortality was 0.26% after DPPHR and 1.31% after PD. After a follow-up of a mean of 45.8 months, 4.11% of the patients with IPMN/MCN developed recurrent tumor, of whom only four succumbed to recurrent tumor at the resection margin. Of 89 patients with PNETs, 2 patients (2.3%) experienced a metastatic recurrence. In-hospital mortality after DPPHR for PNETs was 0%. For patients suffering from premalignant CNs, benign PNETs, or low malignant PATs, DPPHR ensures long-term cure and is associated with a significantly lower risk for surgery-associated complications and low in-hospital mortality when compared with PD.
Limitations
This systemic review and meta-analysis has several limitations. Generally, the inclusion of cohort studies based on a small number of patients increases the risk of bias and limits the conclusions. A total of 5 of the 14 studies used for meta-analysis were retrospective, controlled cohort studies. The comparison of the results of DPPHRt and PD was published, with one exception, in the past 11 years. However, the data from studies of the review group span a reporting period of 27 years. The inclusion of non-comparative studies is of limited evidence.
The incomplete reporting of standard criteria for histopathologic differentiation, and of metrics for postoperative outcome in both groups (DPPHR and PD), limits the conclusions. The results of randomized controlled trials are warranted to establish high-quality clinical evidence regarding the advantages and limitations of DPPHRt compared with PD and the use of DPPHR compared with tumor enucleation.
References
Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138–45.
Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–75.
Sahora K, Fernández-del Castillo C, Dong F, Marchegiani G, Thayer SP, Ferrone CR, et al. Not all mixed-type intraductal papillary mucinous neoplasms behave like main-duct lesions: implications of minimal involvement of the main pancreatic duct. Surgery. 2014;156:611–21.
Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97.
Wang P, Wei J, Wu J, et al. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas: a single institution experience with 97 cases. Pancreatology. 2018;18:415–9.
Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Rel Can. 2008;2008(15):409–27.
Mintziras I, Keck T, Werner J, Fichtner-Feigl S, Wittel U, Senninger N, et al. Implementation of current ENETS guidelines for surgery of small (≤ 2 cm) pancreatic neuroendocrine neoplasms in the German surgical community: an analysis of the prospective DGAV StuDoQ| Pancreas registry. World J Surg. 2019;43:175–82.
Barnes SA, Lillemoe KD, Kaufman HS, et al. Pancreaticoduodenectomy for benign disease. Am J Surgery. 1996;171:131–6.
Newhook TE, LaPar DJ, Lindberg JM, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and mortality of pancreaticoduodenectomy for benign and premalignant pancreatic neoplasms. J Gastrointest Surg. 2015;19:1072–7.
Mavroeidis VK, Russell TB, Clark J, et al. 2023 Pancreatoduodenectomy for suspected malignancy: nonmalignant histology confers increased risk of serious morbidity. Ann R Coll Surg Engl. 2023;105:446–54.
Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide in-hospital mortality following pancreatic surgery in Germany is higher than anticipated. Ann Surg. 2016;264:1082–90.
Liu Z, Peneva IS, Evison F, et al. Ninety day mortality following pancreatoduodenectomy in England: has the optimum centre volume been identified? HPB. 2018;20:1012–20.
Reid-Lombardo KM, Thomsen K, Harmsen WS, Farnell MB. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007;11:1704–11.
Narayanan S, Martin AN, Turrentine FE, Bauer TW, Adams RB, Zaydfudim VM. Mortality after pancreaticoduodenectomy: assessing early and late causes of patient death. J Surg Res. 2018;231:304–8.
Beger HG, Mayer B, Poch B. Long-term metabolic morbidity and steatohepatosis following standard pancreatic resections and parenchyma-sparing, local extirpations for benign tumor - A systematic review and meta-analysis. Ann Surg. 2022;275:54–66.
Thomas AS, Huang Y, Kwon W, et al. Prevalence and risk factors for pancreatic insufficiency after partial pancreatectomy. J Gastrointest Surg. 2022;26:1425–35.
Talamini MA, Moesinger R, Yeo CJ, et al. Cystadenomas of the pancreas: is enucleation an adequate operation? Ann Surg. 1998;227:896–903.
Beger HG, Siech M, Poch B, Mayer B, Schoenberg MH. Limited surgery for benign tumors of the pancreas: a systematic review. World J Surg. 2015;39:1557–66.
Iacono C, Verlato G, Ruzzenente A, et al. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873–85.
Fujii T, Kanda M, Kodera Y, et al. Comparison of pancreatic head resection with segmental duodenectomy and pylorus-preserving pancreatoduodenectomy for benign and low-grade malignant neoplasms of the pancreatic head. Pancreas. 2011;40:1258–63.
Asano T, Nakamura T, Noji T, et al. Outcomes of limited resection for patients with intraductal papillary mucinous neoplasm of the pancreas: a single-center experience. Pancreatology. 2020;20:1399–405.
Beger HG, Mayer B, Poch B. Resection of the duodenum causes long-term endocrine and exocrine dysfunction after Whipple procedure for benign tumors-Results of a systematic review and meta-analysis. HPB. 2020;22:809–20.
Moher D, Liberati L, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Ann Intern Med. 2009;151:265–9.
Oxford Center for Evidence-Based Medicine. available at https://www.cebm.net/index.aspx?o=5653 accessed October 2020.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. Clinical Epidemiology 2016. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Habib JR, Kinny-Köster B, Amini N, et al. Predictors, patterns, and timing of recurrence provide insight into the disease biology of invasive carcinomas arising in association with intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2022;26:2311–20.
Fletcher RH, Flechter SW, Flechter GS. Clinical epidemiology: the essentials. Philadelphia: Lipponcott Williams & Wilkins; 2019.
Lu C, Xu B, Mou Y, Zhou Y, ** W, **a T, et al. Laparoscopic duodenum–preserving pancreatic head resection with real-time indocyanine green guidance of different dosage and timing: enhanced safety with visualized biliary duct and its long-term metabolic morbidity. Langenbeck’s Arch Surg. 2022;407:2823–32.
Zhou M, Xu S, Chao D, Wang M, Zhu F, Peng F, et al. Intracapsular approach used in laparoscopic duodenum-preserving total pancreatic head resection for pancreatic head benign or low-grade malignant tumors. Langenbeck’s Arch Surg. 2022;407:3851–8.
Hong D, Cheng J, Wu W, Liu X, Zheng X. How to perform total laparoscopic duodenum-preserving pancreatic head resection safely and efficiently with innovative techniques. Ann Surg Oncol. 2021;28:3209–16.
Lin X, Zhang J, **e F, Hu Z, He Q, Long Q, et al. Laparoscopic duodenum-preserving total pancreatic head resection in benign and low-grade malignant tumors. Frontiers in Surg. 2013;17(3):126.
Cai Y, Zheng Z, Gao P, Li Y, Peng B. Laparoscopic duodenum-preserving total pancreatic head resection using real-time indocyanine green fluorescence imaging. Surg Endosc. 2021;35:1355–61.
Cao J, Li GL, Wei JX, et al. Laparoscopic duodenum-preserving total pancreatic head resection: a novel surgical approach for benign or low-grade malignant tumors. Surg Endosc. 2019;33:633–8.
Snajdauf J, Rygl M, Petru O, et al. Indications and outcomes of duodenum-preserving resection of the pancreatic head in children. Pediatr Surg Int. 2019;35:449–55.
Milanetto AC, Lico V, Allagio R, Pedrazzoli S, Pasquali C. Duodenum-preserving pancreatic head resection for treatment of neuroendocrine pancreatic tumors of the head of the pancreas. Pancreatology. 2016;16:S65–6.
Thomas E, Matsuoka L, Alexopoulos S, Selby R, Parekh D. Laparoscopic hand-assisted parenchymal-sparing resections for presumed side-branch intraductal papillary mucinous neoplasms. J Laparoend Adv Surg Tech. 2015;25:668–71.
Kozlov IA, Smirnov AV, Chzhao AV. Duodenum-preserving pancreatic head resection and resection of the head of the pancreas combined with segmental duodenectomy. HPB. 2014;16(Suppl. 2):594–5.
Suzuki R, Hatori T, Suzuki S, et al. The treatment strategy of non-functioning pancreatic neuroendocrine tumor. Pancreatology. 2013;4:S74–5.
Tsuchikawa T, Hirano S, Tanaka E, et al. Modified duodenum-preserving pancreas head resection for low-grade malignant lesion in the pancreatic head. Pancreatology. 2013;13:170–4.
Nakagohri T, Kinoshita T, Konishi M, et al. Inferior head resection of the pancreas for intraductal papillary mucinous neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:798–802.
Beger HG, Gansauge F, Siech M, Schwarz M, Poch B. Duodenum-preserving total pancreatic head resection for cystic neoplastic lesions in the head of the pancreas. J Hepatobiliary Pancreat Surg. 2008;15:149–56.
**ong JX, Wang CY, Tao J, Zhang SH. Indication and choice of operation technique for duodenum-preserving resection of pancreatic head: 22 cases reports. Zhonghua wai ke za zhi [Chin J Surg]. 2007;45:24–6.
Fernández-Cruz L, Olvera C, López-Boado MA, Bollo J, Romero J, Comas J. Organ-preserving resection of the pancreaticoduodenal region in the treatment of intraductal papillary mucinous tumors. Cir Esp. 2006;80:295–300.
Ito K, Takada T. Duodenum preservation in pancreatic head resection to maintain pancreatic exocrine function (determined by pancreatic function diagnostant test and cholecystokinin secretion). J Hepatobiliary Pancreat Surg. 2005;12:123–8.
Murakami Y, Uemura K, Yokoyama Y, et al. Pancreatic head resection with segmental duodenectomy for intraductal papillary mucinous tumors of the pancreas. J Gastrointest Surg. 2004;8:713–9.
Hirano S, Kondo S, Ambo Y, et al. Outcome of duodenum-preserving resection of the head of the pancreas for intraductal papillary-mucinous neoplasm. Dig Surg. 2004;21:242–5.
Takada T, Yasuda H, Amano H, Yoshida M. A duodenum-preserving and bile duct-preserving total pancreatic head resection with associated pancreatic duct-to-duct anastomosis. J Gastrointest Surg. 2004;8:220–4.
Isaji S, Kawarada Y. Pancreatic head resection with second-portion duodenectomy for benign lesions, low-grade malignancies, and early stage carcinomas involving the pancreatic head region. Am J Surg. 2001;181:172–6.
Naritomi G, Tanaka M, Matsunaga H, Yokohata K, Ogawa Y, Chijiiwa K, et al. Pancreatic head resection with and without preservation of the duodenum: different postoperative gastric motility. Surgery. 1996;120:831–7.
Imaizumi T, Hanyu F, Suzuki M, Nakasako T, Harada N, Hatori T. Clinical experience with duodenum-preserving total resection of the head of the pancreas with pancreaticocholedochoduodenostomy. J Hepatobiliary Pancreat Surg. 1995;2:38–44.
Harada N. Digestive functions and secretion of gastrointestinal hormones after duodenum-preserving pancreas head resection. Jpn J Gastroenterol Surg. 1994;27:781–8.
Liu W, Peng B. Laparoscopic duodenum-preserving total pancreatic head resection versus laparoscopic pancreaticoduodenectomy for pancreatic-head intraductal papillary mucinous neoplasm. Asian J Surg. 2022;46(6):2293–8.
Sun YH, Ding N, Cheng K, Lin H, Xu JQ, Chen QL. Comparative analysis of duodenum-preserving pancreatic head resection and pancreaticoduodenectomy. Chin Med J. 2020;133:2112–3.
Chen X, Chen W, Zhang Y, An Y, Zhang X. Short-term outcomes of laparoscopic duodenum-preserving total pancreatic head resection compared with laparoscopic pancreaticoduodenectomy for the management of pancreatic-head benign or low-grade malignant lesions. Med Sci Monitor. 2020;26:e927248–51.
Qin H, Yang S, Yang, et al. Duodenum-preserving pancreas head resection in the treatment of pediatric benign and low-grade malignant pancreatic tumors. HPB. 2020;22:306–11.
Jiang Y, ** JB, Zhan Q, et al. Robot-assisted duodenum-preserving pancreatic head resection with pancreaticogastrostomy for benign or premalignant pancreatic head lesions: a single-centre experience. Int J Med Robot. 2018;14:e1903.
Li Y, Wu W, Zhang T, Liao Q, Zhao Y, Dai M. Comparison of long-term benefits of organ-preserving pancreatectomy techniques for benign or low-grade malignant tumors at the pancreatic head. Medicine (Baltimore). 2017;96(51):e9420.
Perinel J, Adham M. Short-and long-term outcomes of pancreatectomy with or without biliary tract and duodenum preservation for benign and borderline neoplasms. Dig Surg. 2014;31:233–41.
Liu JZ, Huang XY, Wang HC, Zhen Q. Duodenum-preserving pancreatic head resection versus pancreaticoduodenectomy for benign pancreatic neoplasms. HPB. 2013;15(Suppl. 2):540.
Gong DJ, Zhang JM, Mao GJ, et al. Duodenum-preserving pancreatic head resection vs. pancreatoduodenectomy for benign lesions and low-grade malignancies of the pancreatic head. Hepatogastroenterology. 2013;60:19–22.
Pedrazzoli S, Canton SA, Sperti C. Duodenum-preserving versus pylorus-preserving pancreatic head resection for benign and premalignant lesions. J Hepatobiliary Pancreat Sci. 2011;18:94–102.
Busquets J, Fabregat J, Borobia FG, et al. Organ-preserving surgery for benign lesions and low-grade malignancies of the pancreatic head: a matched case-control study. Surg Today. 2010;40:125–31.
Horiguchi A, Miyakawa S, Ishihara S, et al. Surgical design and outcome of duodenum-preserving pancreatic head resection for benign or low-grade malignant tumors. J Hepatobiliary Pancreat Sci. 2010;17:792–7.
Lee SE, Jang JY, Hwang DW, Lee KU, Kim SW. Clinical efficacy of organ-preserving pancreatectomy for benign or low-grade malignant potential lesion. J Korean Med Sci. 2010;25(1):97–103.
Beger HG, Mayer B, Poch B. Duodenum-preserving pancreatic head resection for benign and premalignant tumors—A systematic review and meta-analysis of surgery-associated morbidity. J Gastrointest Surg. 2023. https://doi.org/10.1007/s11605-023-05789-4.
Tanaka M, Fernández-del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–53.
European Study Group on Cystic Tumours of the pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789-804.
Vege SS, Ziring B, Jain R, Moayyedi P, Adams MA, Dorn SD, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22.
Vaalavuo Y, Siiki A, Antila A, Rinta-Kiikka I, Sand J, Laukkarinen J. The European evidence-based guidelines on pancreatic cystic neoplasms (PCN) in clinical practice: the development of relative and absolute indications for surgery during prospective IPMN surveillance. Pancreatology. 2020;20:1393–8.
Maker AV, Carrara S, Jamieson NB, et al. Cyst fluid biomarkers for intraductal papillary mucinous neoplasms of the pancreas: a critical review from the international expert meeting on pancreatic branch-duct-intraductal papillary mucinous neoplasms. J Am Coll Surg. 2015;220:243–53.
Tjaden C, Sandini M, Mihaljevic AL, et al. Risk of the watch-and-wait concept in surgical treatment of intraductal papillary mucinous neoplasm. JAMA Surg. 2021;156:818–25.
Salvia R, Burelli A, Perri G, Marchegiani G. State-of-the-art surgical treatment of IPMNs. Langenbeck’s Arch Surg. 2021;406:2633–43.
Asano Y, Kato H, Arakawa S, Ito M, Nagakawa T, Nakao A, et al. Clinical outcomes of organ-preserving pancreatectomy for benign or low-grade malignant pancreatic tumors: a multicenter nationwide survey in Japan. J Hepatobiliary Pancreat Sci. 2022;29(8):898–910.
Hirono S, Shimizu Y, Ohtsuka T, et al. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol. 2020;55:86–99.
Remotti HE, Winner M, Saif MW. Intraductal papillary mucinous neoplasms of the pancreas: clinical surveillance and malignant progression, multifocality and implications of a field-defect. JOP. 2012;13(2):135–8.
Fischer CG, Guthrie VB, Braxton AM, Zheng L, Wang P, Song Q, et al. Intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology. 2019;157:1123–37.
Koh YX, Zheng HL, Chok AY, Tan CS, Wyone W, Lim TK, et al. Systematic review and meta-analysis of the spectrum and outcomes of different histologic subtypes of noninvasive and invasive intraductal papillary mucinous neoplasms. Surgery. 2015;157(3):496.
Noë M, Niknafs N, Fischer CG, Hackeng WM, Beleva Guthrie V, Hosoda W, et al. Genomic characterization of malignant progression in neoplastic pancreatic cysts. Nature Comm. 2020;11:4085.
Kang MJ, Jang JY, Lee KB, Chang YR, Kwon W, Kim SW. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: implications for postoperative surveillance. Ann Surg. 2014;260(2):356–63.
Amini N, Habib JR, Blair A, Rezaee N, Kinny-Köster B, Cameron JL, et al. Invasive and noninvasive progression after resection of noninvasive intraductal papillary mucinous neoplasms. Ann Surg. 2022;276(2):370–7.
Miller JR, Meyer JE, Waters JA, Al-Haddad M, DeWitt J, Sherman S, et al. Outcome of the pancreatic remnant following segmental pancreatectomy for non-invasive intraductal papillary mucinous neoplasm. HPB. 2011;13:759–66.
Rezaee N, Barbon C, Zaki A, He J, Salman B, Hruban RH, et al. Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB. 2016;18(3):236–46.
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103(2):153–71.
Andreasi V, Muffatti F, Guarneri G, Falconi M, Partelli S. Surgical principles in the management of pancreatic neuroendocrine neoplasms. Curr Treat Options Oncol. 2020;21:1–14.
Finkelstein P, Sharma R, Picado O, Gadde R, Stuart H, Ripat C, et al. Pancreatic neuroendocrine tumors (panNETs): analysis of overall survival of nonsurgical management versus surgical resection. J Gastroint Surg. 2017;21:855–66.
Beane JD, Borrebach JD, Billderback A, Onuma AE, Adam MA, Zureikat AH, et al. Small pancreatic neuroendocrine tumors: resect or enucleate? Am J Surg. 2021;222(1):29–34.
Chivukula SV, Tierney JF, Hertl M, Poirier J, Keutgen XM. Operative resection in early stage pancreatic neuroendocrine tumors in the United States: are we over-or undertreating patients? Surgery. 2020;167:180–6.
Sallinen VJ, Le Large TY, Tieftrunk E, et al. & Pancreas 2000 research group. Prognosis of sporadic resected small (≤ 2 cm) nonfunctional pancreatic neuroendocrine tumors–a multi-institutional study. HPB 2018;20:251-259.
Duconseil P, Marchese U, Ewald J, et al. A pancreatic zone at higher risk of fistula after enucleation. World J Surg Oncol. 2018;16:1–9.
Heeger K, Falconi M, Partelli S, Waldmann J, Crippa S, Fendrich V, Bartsch DK. Increased rate of clinically relevant pancreatic fistula after deep enucleation of small pancreatic tumors. Langenbeck’s Arch Surg. 2014;399:315–21.
Michalski CW, Liu B, Heckler M, Roth S, Sun H, Heger U, et al. Underutilization of surgery in periampullary cancer treatment. J Gastrointest Surgery. 2019;23:959–65.
Schirmacher P, Büchler MW. Ampullary adenocarcinoma–differentiation matters. BMC Cancer. 2008;8:1–2.
Wittekind C, Tannapfel A. Adenoma of the papilla and ampulla–premalignant lesions? Langenbeck’s Arch Surg. 2001;386:172–5.
Acknowledgement
Writing assistance was financially supported by the German Foundation for the Fight against Pancreatic Cancer (Grant No. 3/2014).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2024_15222_MOESM1_ESM.pdf
Supplementary file1 Fig. S2 Supplementary file: (DPPHR type I/II/III); (A) type I: partial pancreatic head resection (DPPHRp) - reconstruction with side-to-side pancreaticojejunostomosis, (B) type II: total DPPHR (DPPHRt) - with preservation of the duodenum and the intrapancreatic common bile duct; reconstruction using the first jejunal loop, (C) type III: reconstruction after DPPHRt+sd with segment resection of the peripapillary duodenum and resection of the intrapancreatic common bile duct (PDF 181 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beger, H.G., Mayer, B. & Poch, B. Long-Term Oncologic Outcome following Duodenum-Preserving Pancreatic Head Resection for Benign Tumors, Cystic Neoplasms, and Neuroendocrine Tumors: Systematic Review and Meta-analysis. Ann Surg Oncol 31, 4637–4653 (2024). https://doi.org/10.1245/s10434-024-15222-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15222-y