Abstract
Background
Pathological response is a critical factor in predicting long-term survival of patients with esophageal cancer after preoperative therapy. However, the validity of using pathological response as a surrogate for overall survival (OS) for esophageal cancer has not yet been established. In this study, a literature-based meta-analysis was conducted to evaluate pathological response as a proxy endpoint for survival in esophageal cancer.
Methods
Three databases were systematically searched to identify relevant studies investigating neoadjuvant treatment for esophageal cancer. The correlation between pathological complete response (pCR) and OS were assessed using a weighted multiple regression analysis at the trial level, and the coefficient of determination (R2) was calculated. The research design and histological subtypes were considered in the performance of subgroup analysis.
Results
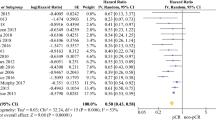
In this meta-analysis, a total of 40 trials, comprising 43 comparisons and 55,344 patients were qualified. The surrogacy between pCR and OS was moderate (R2 = 0.238 in direct comparison, R2 = 0.500 for pCR reciprocals, R2 = 0.541 in log settings). pCR could not serve as an ideal surrogate endpoint in randomized controlled trials (RCTs) (R2 = 0.511 in direct comparison, R2 = 0.460 for pCR reciprocals, R2 = 0.523 in log settings). A strong correlation was observed in studies comparing neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy (R2 = 0.595 in direct comparison, R2 = 0.840 for pCR reciprocals, R2 = 0.800 in log settings).
Conclusions
A lack of surrogacy of pathological response for long-term survival at trial level is established in this study. Hence, caution should be exercised when using pCR as the primary endpoint in neoadjuvant studies for esophageal cancer.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The data are available online, and additional materials are available by contacting with authors.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology. 2015;149(2). doi:https://doi.org/10.1053/j.gastro.2015.04.053.
Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–83. https://doi.org/10.6004/jnccn.2019.0033.
Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39(18):1995–2004. https://doi.org/10.1200/JCO.20.03614.
Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339(27):1979–84.
Kim MM, Allen P, Gonzalez-Angulo AM, et al. Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol. 2013;24(8):1999–2004. https://doi.org/10.1093/annonc/mdt131.
Passot G, You BT, Boschetti G, et al. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21(8):2608–14. https://doi.org/10.1245/s10434-014-3647-0.
Corsini EM, Weissferdt A, Pataer A, et al. Pathological nodal disease defines survival outcomes in patients with lung cancer with tumour major pathological response following neoadjuvant chemotherapy. Eur J Cardiothorac Surg. 2021;59(1):100–8. https://doi.org/10.1093/ejcts/ezaa290.
US Department of Health and Human Services. Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. Accessed 2020-07-29, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathological-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use
Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87(2). doi:https://doi.org/10.1016/j.athoracsur.2008.11.001
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Buyse M, Burzykowski T, Michiels S, Carroll K. Individual- and trial-level surrogacy in colorectal cancer. Stat Methods Med Res. 2008;17(5):467–75. https://doi.org/10.1177/0962280207081864.
**e W, Halabi S, Tierney JF, et al. A systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. JNCI Cancer Spectr. 2019;3(1):pkz002. doi:https://doi.org/10.1093/jncics/pkz002
Barbour AP, Walpole ET, Mai GT, et al. Preoperative cisplatin, fluorouracil, and docetaxel with or without radiotherapy after poor early response to cisplatin and fluorouracil for resectable oesophageal adenocarcinoma (AGITG DOCTOR): results from a multicentre, randomised controlled phase II trial. Ann Oncol. 2020;31(2):236–45. https://doi.org/10.1016/j.annonc.2019.10.019.
Stiles BM, Kamel MK, Harrison SW, et al. Neoadjuvant therapy for locally advanced esophageal cancer should be targeted to tumor histology. Ann Thorac Surg. 2019;107(1):187–93. https://doi.org/10.1016/j.athoracsur.2018.07.089.
Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47(3):354–60. https://doi.org/10.1016/j.ejca.2010.09.009.
Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017;18(3):357–70. https://doi.org/10.1016/S1470-2045(17)30043-8.
Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol. 2017;18(9):1249–60. https://doi.org/10.1016/S1470-2045(17)30447-3.
Vos EL, Carr RA, Hsu M, et al. Prognosis after neoadjuvant chemoradiation or chemotherapy for locally advanced gastro-oesophageal junctional adenocarcinoma. Br J Surg. 2021;108(11):1332–40. https://doi.org/10.1093/bjs/znab228.
Al-Sukhni E, Gabriel E, Attwood K, Kukar M, Nurkin SJ, Hochwald SN. No survival difference with neoadjuvant chemoradiotherapy compared with chemotherapy in resectable esophageal and gastroesophageal junction adenocarcinoma: results from the National Cancer Data Base. J Am Coll Surg. 2016;223(6). doi:https://doi.org/10.1016/j.jamcollsurg.2016.09.002
Visser E, Edholm D, Smithers BM, et al. Neoadjuvant chemotherapy or chemoradiotherapy for adenocarcinoma of the esophagus. J Surg Oncol. 2018;117(8):1687–96. https://doi.org/10.1002/jso.25089.
Ho F, Torphy RJ, Friedman C, et al. Induction chemotherapy plus neoadjuvant chemoradiation for esophageal and gastroesophageal junction adenocarcinoma. Ann Surg Oncol. 2021;28(12):7208–18. https://doi.org/10.1245/s10434-021-09999-5.
Macedo FI, Mesquita-Neto JW, Kelly KN, et al. Utility of radiation after neoadjuvant chemotherapy for surgically resectable esophageal cancer. Ann Surg Oncol. 2020;27(3):662–70. https://doi.org/10.1245/s10434-019-07788-9.
von DÃbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. 2019;32(2). doi:https://doi.org/10.1093/dote/doy078
Tiesi G, Park W, Gunder M, et al. Long-term survival based on pathologic response to neoadjuvant therapy in esophageal cancer. J Surg Res. 2017;216:65–72. https://doi.org/10.1016/j.jss.2017.03.022.
Zhang G, Zhang C, Sun N, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the National Cancer Center in China. J Cancer Res Clin Oncol. 2022;148(4):943–54. https://doi.org/10.1007/s00432-021-03659-7.
Yoon HH, Ou F-S, Soori GS, et al. Induction versus no induction chemotherapy before neoadjuvant chemoradiotherapy and surgery in oesophageal adenocarcinoma: a multicentre randomised phase II trial (NCCTG N0849 [Alliance]). Eur J Cancer. 2021;150:214–23. https://doi.org/10.1016/j.ejca.2021.03.025.
Wundsam HV, Doleschal B, Prommer R, et al. Clinical outcome in patients with carcinoma of the esophagogastric junction treated with neoadjuvant radiochemotherapy or perioperative chemotherapy: a two-center retrospective analysis. Oncology. 2020;98(10):706–13. https://doi.org/10.1159/000507706.
Ajani JA, **ao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2013;24(11):2844–9. https://doi.org/10.1093/annonc/mdt339.
Miller JA, Groman A, Yendamuri S, Hennon M. Radiation with neoadjuvant chemotherapy does not improve outcomes in esophageal squamous cell cancer. J Surg Res. 2019;236:259–65. https://doi.org/10.1016/j.jss.2018.11.052.
Lyu J, Liu T, Li T, et al. Comparison of efficacy, safety, and costs between neoadjuvant hypofractionated radiotherapy and conventionally fractionated radiotherapy for esophageal carcinoma. Cancer Med. 2019;8(8):3710–8. https://doi.org/10.1002/cam4.2250.
** J, Liao Z, Zhang Z, et al. Induction chemotherapy improved outcomes of patients with resectable esophageal cancer who received chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys. 2004;60(2):427–36.
Haisley KR, Hart KD, Nabavizadeh N, et al. Neoadjuvant chemoradiotherapy with concurrent cisplatin/5-fluorouracil is associated with increased pathologic complete response and improved survival compared to carboplatin/paclitaxel in patients with locally advanced esophageal cancer. Dis Esophagus. 2017;30(7):1–7. https://doi.org/10.1093/dote/dox015.
Ji KSY, Thomas SM, Roman SA, et al. Low- vs. high-dose neoadjuvant radiation in trimodality treatment of locally advanced esophageal cancer. J Gastrointest Surg. 2019;23(5):885–94. https://doi.org/10.1007/s11605-018-4007-3.
Morgan MA, Lewis WG, Crosby TDL, et al. Prospective cohort comparison of neoadjuvant chemoradiotherapy versus chemotherapy in patients with oesophageal cancer. Br J Surg. 2007;94(12):1509–14.
Yamasaki M, Yasuda T, Yano M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28(1):116–20. https://doi.org/10.1093/annonc/mdw439.
** M, Liao Z, Deng W, et al. A Prognostic scoring model for the utility of induction chemotherapy prior to neoadjuvant chemoradiotherapy in esophageal cancer. J Thorac Oncol. 2017;12(6):1001–10. https://doi.org/10.1016/j.jtho.2017.03.017.
** M, Zhang P, Zhang L, et al. Comparing docetaxel plus cisplatin versus fluorouracil plus cisplatin in esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Jpn J Clin Oncol. 2017;47(8):683–9. https://doi.org/10.1093/jjco/hyx060.
Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–90. https://doi.org/10.1016/j.ejca.2017.04.027.
Author XXX
Mukherjee S, Hurt C, Radhakrishna G, et al. Oxaliplatin/capecitabine or carboplatin/paclitaxel-based preoperative chemoradiation for resectable oesophageal adenocarcinoma (NeoSCOPE): long-term results of a randomised controlled trial. Eur J Cancer. 2021;153:153–61. https://doi.org/10.1016/j.ejca.2021.05.020.
Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–40.
Mauguen A, Pignon J-P, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013;14(7):619–26. https://doi.org/10.1016/S1470-2045(13)70158-X.
Oba K, Paoletti X, Alberts S, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: a meta-analysis. J Natl Cancer Inst. 2013;105(21):1600–7. https://doi.org/10.1093/jnci/djt270.
Ajani JA, Leung L, Singh P, et al. Disease-free survival as a surrogate endpoint for overall survival in adults with resectable esophageal or gastroesophageal junction cancer: a correlation meta-analysis. Eur J Cancer. 2022;170:119–30. https://doi.org/10.1016/j.ejca.2022.04.027.
Ronellenfitsch U, Jensen K, Seide S, et al. Disease-free survival as a surrogate for overall survival in neoadjuvant trials of gastroesophageal adenocarcinoma: Pooled analysis of individual patient data from randomised controlled trials. Eur J Cancer. 2019;123:101–11. https://doi.org/10.1016/j.ejca.2019.10.001.
Al-Kaabi A, van der Post RS, van der Werf LR, et al. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long-term outcome of esophageal cancer: a population-based study. Acta Oncol. 2021;60(4):497–504. https://doi.org/10.1080/0284186X.2020.1870246.
Chao Y-K, Chen H-S, Wang B-Y, Hsu P-K, Liu C-C, Wu S-C. Factors associated with survival in patients with oesophageal cancer who achieve pathological complete response after chemoradiotherapy: a nationwide population-based study. Eur J Cardiothorac Surg. 2017;51(1):155–9. https://doi.org/10.1093/ejcts/ezw246.
Blum Murphy M, **ao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival–The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123(21):4106–13. https://doi.org/10.1002/cncr.30953.
Conforti F, Pala L, Sala I, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375:e066381. https://doi.org/10.1136/bmj-2021-066381.
Klevebro F, Alexandersson von DÃbeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27(4):660–667. doi:https://doi.org/10.1093/annonc/mdw010
Rose BS, Winer EP, Mamon HJ. Perils of the pathologic complete response. J Clin Oncol. 2016;34(33):3959–62. https://doi.org/10.1200/JCO.2016.68.1718.
Chiang AC, Massaguà J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–23. https://doi.org/10.1056/NEJMra0805239.
Klein CA. Selection and adaptation during metastatic cancer progression. Nature. 2013;501(7467):365–72. https://doi.org/10.1038/nature12628.
Colori A, Hiley C. The interaction of preexisting cardiac dysfunction and heart dose from radical radiotherapy on all-cause mortality in locally advanced NSCLC. J Thorac Oncol. 2023;18(1):14–6. https://doi.org/10.1016/j.jtho.2022.10.017.
Burstein HJ. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med. 2020;383(26):2557–70. https://doi.org/10.1056/NEJMra1307118.
Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31(25):2973–84. https://doi.org/10.1002/sim.5403.
Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics. 1998;54(3):1014–29.
Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1(1):49–67.
Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol. 2023;34(2):163–72. https://doi.org/10.1016/j.annonc.2022.10.508.
von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31(29):3623–30. https://doi.org/10.1200/JCO.2012.45.0940.
Rizvi FH, Syed AA, Khattak S, Rizvi SSH, Kazmi SA, Khan MQ. Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: a retrospective cohort study. Int J Surg. 2014;12(6):621–5. https://doi.org/10.1016/j.ijsu.2014.04.014.
Wang H, Tang H, Fang Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2021;156(5):444–51. https://doi.org/10.1001/jamasurg.2021.0133.
RodrÃguez-Ruiz MAE, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39(8):644–655. doi:https://doi.org/10.1016/j.it.2018.06.001
Ruhstaller T, Thuss-Patience P, Hayoz S, et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: a randomized, open-label, phase III trial (SAKK 75/08). Ann Oncol. 2018;29(6):1386–93. https://doi.org/10.1093/annonc/mdy105.
Acknowledgement
This work is supported by the National Natural Science Foundation of China (81400681), China Postdoctoral Science Foundation Grant (2018M631394), and Science and Technology Innovation Plan of Shanghai Science and Technology Commission (22Y11907200). The funding agencies had no role in study design, collection and analyses of data, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
FS and XY searched the databases, and extracted and analyzed the data. JY, FS and XY screened and selected the qualified literatures. YS and LT designed and conceived the study. FS, and YS formulated the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2023_13778_MOESM3_ESM.png
Supplementary Figure 2. Correlations between effects of esophageal cancer treatment on event free survival (EFS) and overall survival (OS) and reverse pathological response in the main analysis. Each circle represents a trial, and the surface area of the circle is proportional to the number of events observed in the corresponding trial. (A) OS and reciprocals of complete pathological response (rpCR) in main analysis; (B) OS and rpCR in prospective studies; (C) OS and rpCR in retrospective studies; (D) OS and rpCR in adenocarcinoma; (E) OS and rpCR in squamous cell carcinoma; (F) OS and reciprocals of major pathological response (rmPR) in main analysis; (G) EFS and rmPR in main analysis Solid lines represent weighted regression lines and dotted lines represent the 95% prediction interval. (PNG 59 kb)
10434_2023_13778_MOESM4_ESM.png
Supplementary Figure 3. Correlations between effects of esophageal cancer treatment on event free survival (EFS) and overall survival (OS) and major pathological response (mPR) in the main analysis. Each circle represents a trial, and the surface area of the circle is proportional to the number of events observed in the corresponding trial. Solid lines represent weighted regression lines and dotted lines represent the 95% prediction interval. (PNG 60 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, F., Yang, X., Yin, J. et al. Validity of Using Pathological Response as a Surrogate for Overall Survival in Neoadjuvant Studies for Esophageal Cancer: A Systematic Review and Meta-analysis. Ann Surg Oncol 30, 7461–7471 (2023). https://doi.org/10.1245/s10434-023-13778-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13778-9