Abstract
Purpose
The optimal mode of neoadjuvant treatment for esophageal squamous cell carcinoma (ESCC) has not been well characterized. Our study compared neoadjuvant chemotherapy (NCT) with neoadjuvant chemoradiotherapy (NCRT) for patients with ESCC.
Methods
Data from ESCC patients receiving NCRT or NCT combined with esophagectomy between 2010 and 2018 from the National Cancer Center in China were retrospectively collected. Long-term survival, pathological response, and perioperative mortality and morbidity were compared between the NCRT and NCT groups. A Cox proportional hazards model and propensity score matching (PSM) were used to minimize bias due to potential confounding.
Results
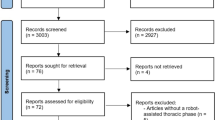
Out of 327 eligible patients with ESCC in our study, 90 patients were identified in each group by PSM. The complete pathologic response (pCR) rate in the NCRT group was markedly higher than that in the NCT group (before PSM: 35.1% vs. 6.0%; after PSM: 38.9% vs. 5.6%; both P < 0.001). The rates of 30-day or 90-day mortality were comparable between the two groups, but the NCRT group had a longer postoperative hospital stay (P < 0.001 before PSM and P = 0.012 after PSM) and more postoperative complications (P < 0.001 before PSM and P = 0.014 after PSM), especially, anastomotic leaks (P = 0.001 before PSM and P = 0.013 after PSM). No significant differences in 5-year overall survival (OS) (P = 0.439) or 5-year relapse-free survival (RFS) (P = 0.611) were noted between unmatched groups, but the trend favored NCRT in the propensity score-matched group (77.3% vs. 61.3%; hazard ratio [HR] 1.57; 95% confidence interval [CI] 0.86–2.87; P = 0.141 for OS, and 77.8% vs. 60.5%; HR 1.72; 95% CI 0.95–3.11; P = 0.073 for RFS). Multivariate analysis showed that only ypT and ypN stages were independent predictors of OS before and after PSM (both P < 0.05).
Conclusion
There was no difference in survival between the NCT and NCRT groups, although a trend favored NCRT related to the significantly higher pCR rates. Prospective head-to-head clinical trials to compare these two types of neoadjuvant therapies in ESCC are warranted.


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abnet CC, Arnold M, Wei WQ (2018) Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154:360–373
Ajani JA, D’Amico TA, Bentrem DJ et al (2019) Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 17:855–883
Allum WH, Stenning SP, Bancewicz J et al (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062–5067
Berger AC, Farma J, Scott WJ et al (2005) Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 23:4330–4337
Blom RL, Lagarde SM, van Oudenaarde K et al (2013) Survival after recurrent esophageal carcinoma has not improved over the past 18 Years. Ann Surg Oncol 20:2693–2698
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Burmeister BH, Thomas JM, Burmeister EA et al (2011) Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 47:354–360
Chen C, Yu Z, ** Q et al (2015) Clinical features and risk factors of anastomotic leakage after radical esophagectomy. Zhonghua Wai Ke Za Zhi 53:518–521
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Daxuan H, Xue L, Yuanyuan Y et al (2017) Neoadjuvant chemoradiotherapy versus chemotherapy and surgery for patients with locally advanced esophageal squamous cell carcinoma. Transl Cancer Res 6:346–353
Deng HY, Wang WP, Wang YC et al (2017) Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg 51:421–431
Gao X, Wang Z, Kong C et al (2017) Trends of esophageal cancer mortality in rural china from 1989 to 2013: an age-period-cohort analysis. Int J Environ Res Public Health 14:218
Huang J, Xu J, Chen Y et al (2020) Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 21:832–842
Klevebro F, Alexandersson von Döbeln G, Wang N et al (2016) A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 27:660–667
Launoy G, Bossard N, Castro C et al (2017) Trends in net survival from esophageal cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev 26:S24–S31
Leng X, He W, Yang H et al (2019) Prognostic impact of postoperative lymph node metastases after neoadjuvant chemoradiotherapy for locally advanced squamous cell carcinoma of esophagus: from the results of neocrtec5010, a randomized multicenter study. Ann Surg. https://doi.org/10.1097/SLA.0000000000003727
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic Correlations Cancer 73:2680–2686
Markar SR, Noordman BJ, Mackenzie H et al (2017) Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol 28:519–527
Nakashima Y, Saeki H, Hu Q et al (2018) Neoadjuvant chemotherapy versus chemoradiotherapy for patients with esophageal squamous cell carcinoma. Anticancer Res 38:6809–6814
Petrelli F, Ghidini M, Barni S et al (2019) Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis. Gastric Cancer 22:245–254
Roh S, Iannettoni MD, Keech J et al (2019) Timing of esophagectomy after neoadjuvant chemoradiation therapy affects the incidence of anastomotic leaks. Korean J Thorac Cardiovasc Surg 52:1–8
Samson P, Puri V, Lockhart AC et al (2018) Adjuvant chemotherapy for patients with pathologic node-positive esophageal cancer after induction chemotherapy is associated with improved survival. J Thorac Cardiovasc Surg 156:1725–1735
Shapiro J, van Lanschot JJB, Hulshof MCCM et al (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16:1090–1098
Sihag S, Ku GY, Tan KS et al (2020) (2020) Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg. S0022–5223(20):33192–33195. https://doi.org/10.1016/j.jtcvs.2020.11.106 (Online ahead of print)
Sjoquist KM, Burmeister BH, Smithers BM et al (2011) Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 12:681–692
Stahl M, Walz MK, Stuschke M et al (2009) Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27:851–856
Stahl M, Walz MK, Riera-Knorrenschild J et al (2017) Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer 81:183–190
van den Ende T, Clercq N, van Berge Henegouwen MI et al (2021) Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A Single Arm Phase II Feasibility Trial (PERFECT). Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-4443 (Online ahead of print)
Vande Walle C, Ceelen WP, Boterberg T et al (2012) Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys 82:e513–e519
von Döbeln GA, Klevebro F, Jacobsen AB et al (2019) Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus 32:1–11
Wang J, Wei C, Tucker SL et al (2013) Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 86:885–891
Watanabe M, Otake R, Kozuki R et al (2020) Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 50:12–20
Yang H, Liu H, Chen Y et al (2018) Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 36:2796–2803
Zhao X, Ren Y, Hu Y et al (2018) Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLoS ONE 13:e0202185
Zhou HY, Zheng SP, Li AL et al (2020) Clinical evidence for association of neoadjuvant chemotherapy or chemoradiotherapy with efficacy and safety in patients with resectable esophageal carcinoma (NewEC study). EClinicalMedicine 24:100422
Funding
This work was supported by funds from the National Key Research and Development Program of China [2016YFC1303200].
Author information
Authors and Affiliations
Contributions
JH supervised the project, design, interpretation, manuscript revision, and final approval of the version to be submitted. GCZ, CQZ, NS and QX were involved in concept, data acquisition, analysis and interpretation. GCZ and CQZ wrote the first draft, and revised it critically in light of comments from other authors. GCZ, ZHZ and YJL prepared all the figures and tables. LYX and ZYY reviewed all specimens enrolled in the study. LLF, JWM, YSG and FWT were involved in data acquisition and provided material support. SGG and QX were involved in the manuscript revision and discussion. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The research was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Bei**g, China). Written informed consent was waived because this was a retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, G., Zhang, C., Sun, N. et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the National Cancer Center in China. J Cancer Res Clin Oncol 148, 943–954 (2022). https://doi.org/10.1007/s00432-021-03659-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03659-7