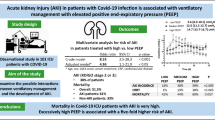
Abstract
Background
Lung perfusion defects, mainly due to endothelial and coagulation activation, are a key contributor to COVID-19 respiratory failure. COVID-19 patients may also develop acute kidney injury (AKI) because of renal perfusion deficit. We aimed to explore AKI-associated factors and the independent prediction of standardized minute ventilation (MV)—a proxy of alveolar dead space—on AKI onset and persistence in COVID-19 mechanically ventilated patients.
Methods
This is a multicenter observational cohort study. We enrolled 157 COVID-19 patients requiring mechanical ventilation and intensive care unit (ICU) admission. We collected clinical information, ventilation, and laboratory data. AKI was defined by the 2012 KDIGO guidelines and classified as transient or persistent according to serum creatinine criteria persistence within 48 h. Ordered univariate and multivariate logistic regression analyses were employed to identify variables associated with AKI onset and persistence.
Results
Among 157 COVID-19 patients on mechanical ventilation, 47% developed AKI: 10% had transient AKI, and 37% had persistent AKI. The degree of hypoxia was not associated with differences in AKI severity. Across increasing severity of AKI groups, despite similar levels of paCO2, we observed an increased MV and standardized MV, a robust proxy of alveolar dead space. After adjusting for other clinical and laboratory covariates, standardized MV remained an independent predictor of AKI development and persistence. d-dimer levels were higher in patients with persistent AKI.
Conclusions
In critically ill COVID-19 patients with respiratory failure, increased wasted ventilation is independently associated with a greater risk of persistent AKI. These hypothesis-generating findings may suggest that perfusion derangements may link the pathophysiology of both wasted ventilation and acute kidney injury in our population.
Similar content being viewed by others
Background
Kidney and lung injuries are common and associated with significant morbidity and mortality in critically ill patients [1, 2]. Patients with acute kidney injury (AKI) are more likely to require mechanical ventilation [1, 2], whereas an increased risk for AKI was observed in patients with acute respiratory failure or acute respiratory distress syndrome (ARDS) [3]. The co-existence of AKI, even mild or moderate, with ARDS was associated with prolonged ICU and hospital stay, prolonged duration of mechanical ventilation, and higher hospital mortality in a secondary analysis [4] of the LUNG SAFE study [5]. The recent COVID-19 pandemic sparked once again the interest about the lung—kidney crosstalk in critically ill patients. Despite initial reports [6], it has been observed that AKI frequency reaches 30% among patients with COVID-19 requiring ICU admission and mechanical ventilation [7, 8].
Several mechanisms explain how lung injury may adversely affect kidney function in the context of “typical” ARDS. First, the reduced cardiac output due to positive pressure ventilation [9, 10] and/or venous stasis [11] may reduce renal blood flow. In the COVID-19 ARDS, the transmission of the pulmonary pressures to the thoracic compartment and its effect on the renal perfusion may be even more pronounced [12, 13], since respiratory system mechanics are often preserved, when compared with typical ARDS [14]. Second, the changes in the partial pressure of oxygen and carbon dioxide may affect renal perfusion by influencing vascular resistance [15]. Finally, in the context of ventilatory-induced lung injury (VILI), biotrauma may lead to systemic inflammation and organ dysfunction via the release of inflammatory cytokines [16, 17]. When considering the interplay between COVID-19-related respiratory and kidney failure, unique features of SARS-CoV-2 infection have to be considered. The SARS-CoV-2 virus interaction with the angiotensin‐converting enzyme 2 (ACE2) receptor, widely expressed on the renal epithelium, could lead to direct damage to the renal parenchyma [18]. Kidney injury could be also systemically mediated by inflammatory cell recruitment, macrophage activation syndrome, and cytokine storm [19]. The systemic inflammation promotes the activation of deranged coagulation pathways, leading to perfusion impairment in renal small vessels. Autopsy studies supported the concept of renal “malperfusion,” reporting glomerular and peritubular capillaries thrombosis associated with glomerular ischemia [20, 21]. In COVID-19 respiratory failure, the presence of perfusion deficits in systemic and pulmonary circulation has been reported [22, 23]. Additionally, coagulopathy has been identified as an independent predictor for pulmonary and systemic thromboembolic events [22]. These findings may suggest a disease-specific AKI characterized by thromboembolic manifestations, coagulation impairment, and endothelial cell swelling with foamy degeneration resulting in endothelialitis [21, 22]. Interestingly, in COVID-19 respiratory failure, the activation of deranged coagulation, as assessed by increasing levels of d-dimer, was associated with a significant alveolar dead space, resulting in an increased respiratory workload [23].
Whether the presence of dead space in COVID-19 respiratory failure—as result of coagulation activation and ventilation-perfusion mismatch—may explain kidney malperfusion and the development of AKI is yet to be demonstrated. We hypothesize that acute kidney and lung injury may be driven by common and linked pathophysiologic processes including coagulation activation and perfusion deficit.
In this multicenter retrospective study, we aimed to investigate whether laboratory parameters suggestive of inflammation and coagulation activation, along with ventilatory indices indicating the severity of lung injury and increased ventilatory load are associated with AKI development and persistence [24].
Methods
Study design and data collection
In this multi-center international retrospective observational study, we enrolled 161 consecutive adult patients, with a diagnosis by real-time PCR of COVID-19 pneumonia requiring mechanical ventilation and ICU admission from February to May 2020. Exclusion criteria were age less than 18 years old and pregnancy. The participating centers included IRCSS Fondazione San Gerardo dei Tintori (Monza, Italy), Policlinico San Marco (Zingonia – Bergamo, Italy), and Galway University Hospitals (Galway, Ireland). This research was part of the STORM Study approved by Istituto Nazionale Malattie Infettive Lazzaro Spallanzani, Rome, Italy (Resolution no. 84/2020; NCT04424992). The local ethics committee of Galway University Hospitals, Galway (C.A. 2384), and Policlinico San Marco, Zingonia (Reg. Sperim. N. 118/20), approved the study. Informed consent was waived considering the observational, non-interventional nature of the study.
Demographic, anamnestic, and clinical data of all patients were collected in a dedicated database. Laboratory data, including complete blood count, coagulation panel, liver and kidney function tests, and arterial blood gas analysis, were daily recorded from the day of ICU admission to day 7 and then every 7 days until day 28. Ventilatory settings and ventilatory mechanics parameters were recorded in the same way. The need for adjunctive therapies such as neuromuscular blockade, pronation, inhaled nitric oxide, veno-venous extracorporeal membrane oxygenation (V-V ECMO), and renal replacement therapy (RRT) was recorded. Furthermore, we collected data about ICU outcomes.
We defined and staged AKI by using serum creatinine over 7 days from ICU admission, according to the 2012 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines [25]. In most of the clinical charts, the hourly urinary output was either unavailable or unreliable (e.g., not precisely recorded every hour) due to the pandemic context. For these reasons, we decided to base our AKI definition and classification solely on the creatinine parameter, consistent with literature from the pandemic period [26, 27].
Baseline renal function was defined using serum creatinine within six months prior to ICU admission when available. Otherwise, we estimated serum creation by using the following formula, as reported as 2021 CKD—EPI creatinine [28].
where A and B are 0.7 and − 1.2, respectively, if female and 0.9 and − 1.2 if male. Persistent AKI was defined by the continuance of AKI by serum creatinine criteria, according to the KDIGO guidelines, beyond 48 h from its onset. If a complete and sustained reversal of the AKI episode occurred within 48 h, we categorized the AKI as transient [24]. Patients with chronic kidney disease (CKD) at any stage were excluded from statistical analysis, considering that serum creatine estimate is not reliable in this population and that one of the most significant risk factors for AKI is pre-existing CKD and AKI itself plays a role in both the CKD development and the progression of pre-existing CKD [29].
Statistical analysis
Normality was assessed using the Shapiro-Wilk test, and continuous data are expressed as mean ± SD or median [IQR], as appropriate. Categorical data are expressed as count (proportion). The test for the trend of normally distributed variables across ordered groups was performed by using linear regression, while the trend of non-normally distributed variables was tested by Jonckheere-Terpstra test. Differences in continuous data were assessed by unpaired Student’s T test or U Mann-Whitney test, as appropriate. Categorical data across ordered groups were evaluated via Cochran-Armitage trend test. Chi-square test was performed to compare categorical data between two groups. The effect of the AKI group and the day from the ICU admission on continuous variables was tested using two-way ANOVA. Post hoc analysis was conducted using the Sidak correction method to evaluate the difference in the continuous variable between different AKI groups. The correlations between increasing AKI stages (i.e., No AKI, transient AKI, persistent AKI) and qualitative and quantitative variables were obtained using ordered univariable and multivariable logistic regressions. The factors associated with a more severe AKI stage by using a univariable ordered logistic regression analysis (p < 0.05) and considered clinically meaningful were included in ordered logistic multivariable models. The significance level was set to 5% (two-sided). Stata/MP version 17 (Copyright 1985–2021 StataCorp LLC, College Station, TX, USA), and IBM SPSS Statistics (IBM SPSS Statistics for Macintosh, Version 20.0. Armonk, NY: IBM Corp) were used for the statistical analyses.
Results
Study population
We enrolled 161 patients admitted to the intensive care units of the participating centers from February to May 2020. Eighty-nine (55%) patients were admitted to Monza, 51 (32%) at Zingonia, and 21 (13%) at Galway Hospital. We excluded four patients with chronic kidney disease. Thus, 157 patients were included in the final analysis. All patients presented hypoxemic respiratory failure due to COVID-19 requiring ICU admission and invasive mechanical ventilation. We stratified the population into three groups based on the temporary presence and persistence of AKI during the ICU stay. Seventy-three (46.5%) patients developed AKI. Of these, 10 (15%) showed a transient AKI, whereas in 58 (37%) patients, the AKI was persistent. We observed more patients without AKI in Monza hospital, while a higher incidence of persistent AKI was reported in Zingonia hospital. No differences in demographic characteristics and main comorbidities were observed among the three groups (Table 1).
Renal characteristics
Baseline creatinine was similar among the three groups. As compared with baseline, a trend toward higher levels of creatinine and urea at ICU admission and higher creatinine peak within 7 days in patients with persistent AKI was observed (Table 1). The levels of creatinine remained higher in the persistent AKI group during the first 7 days after ICU admission (Fig. 1). A higher fluid balance in the first 24 h was observed in the persistent AKI group. Among the patients who developed transient and persistent AKI, 2 (13%) and 16 (28%) patients required continuous renal replacement therapy (CRRT), respectively (Table 1).
Creatinine, d-dimer, WBC, and PCT during the first ICU week, stratified in no AKI, transient AKI, and persistent AKI groups. The trend in creatinine, d-dimer, WBC, and PCT over time in patients without AKI (green circles), with transient (blue triangles), and persistent (red squares) AKI. All data represent mean ± SEM. $ refers to the effect of AKI groups on the continuous variable; $$ refers to the effect of day from ICU admission on the continuous variable; $$$ refers to the interaction between AKI groups and day from ICU admission on the continuous variable. ✚P < 0.05 for comparison between no AKI and transient AKI groups; *P < 0.05 for comparison between no-AKI and persistent AKI groups; #P < 0.05 for comparison between transient and persistent AKI groups. Definition of abbreviation. PCT, procalcitonin; WBC, white blood cells
Laboratory data at ICU admission
To investigate a possible role of inflammation in the development of AKI, we analyzed inflammatory markers and coagulation function among the three patients’ groups. White blood cells were higher in patients who developed AKI compared to those who did not, while inflammatory markers and coagulation function were similar among the three groups (Table 2). During the first 7 days of ICU, patients who developed AKI exhibited higher levels of inflammatory markers and a trend toward higher values of white blood cells. A significant effect of AKI groups on d-dimer level and a trend toward more elevated d-dimer level was observed in the persistent AKI patients (Fig. 1). A more severe metabolic impairment was observed in patients with AKI (Table 2).
Respiratory parameters
Upon admission, the PaO2/FiO2 ratio was similar in patients who developed persistent or transient AKI and in patients who did not. The median of PaO2 during the first 7 ICU days was lower in AKI patients. Patients were ventilated with higher median PEEP and FiO2 levels in the first week of ICU stay by moving through AKI classes. The respiratory system mechanics at ICU admission were similar among the groups (Table 2). No difference was observed in arterial CO2 tension at ICU admission, but a significant trend toward a lower-end tidal CO2 on admission and during the first week was reported in AKI groups (Table 2). Furthermore, the median dead space, calculated as the difference between PaCO2 and end-tidal CO2 divided by PaCO2 [30], increased significantly across the three cohorts during the first ICU week (Table 2).
On admission and during the first week of ICU, patients presented a higher minute ventilation from no AKI to higher severity classes of AKI (Fig. 2). The proportion of patients who underwent prone positioning decreased from the no-AKI to the persistent AKI group, while no difference in the use of neuromuscular blocking drugs, inhaled nitric oxide and V-V ECMO was observed in the two groups (Table 2). The overall population of patients who underwent prone position was ventilated with higher PEEP levels than those who did not. Patients who underwent prone position in no-AKI and persistent AKI groups at ICU admission (Additional file 1: Table S1)—as well as during the first ICU week (Additional file 1: Table S2)—were exposed to higher PEEP levels than those who did not.
PaCO2, minute ventilation, and standardized minute ventilation, during the first ICU week, stratified in no AKI, transient AKI, and persistent AKI groups. The trend in PaCO2, minute ventilation, and standardized minute ventilation over time in patients without AKI (green circles), with transient (blue triangles), and persistent (red squares) AKI. All data represent mean ± SEM. $ refers to the effect of AKI groups on the continuous variable; $$ refers to the effect of day from ICU admission on the continuous variable; $$$ refers to the interaction between AKI groups and day from ICU admission on the continuous variable. ✚P < 0.05 for comparison between no-AKI and transient AKI groups; *P < 0.05 for comparison between no-AKI and persistent AKI groups; #P < 0.05 for comparison between transient and persistent AKI groups. Definition of abbreviation. PaCO2, arterial partial pressure of carbon dioxide
Upon dividing AKI subgroups into ordinal quartiles of respiratory load indicators, we noted a significantly higher percentage of subjects develo** persistent AKI in the higher MV and standardized MV quartiles and a trend toward a higher percentage of persistent AKI in the quartiles with higher PaCO2 (Fig. 3). We did not observe any difference in the percentage of persistent AKI patients among the PaO2/FiO2 ratio quartiles (Fig. 4).
Subjects (%) stratified in no AKI/transient AKI and persistent AKI subgroups in ordinal groups of PaCO2, minute ventilation, and standardized minute ventilation. PaCO2 (mmHg): Q1 < 38.7, Q2 ≥ 38.7 - < 45.1, Q3 ≥ 45.1 - < 56.2, Q4 ≥ 56.2; minute ventilation (L/min): Q1 < 8.7, Q2 ≥ 8.7 - < 10.3, Q3 ≥ 10.3 - < 12.0, Q4 ≥ 12.0; standardized minute ventilation (L/min): Q1 < 9.6, Q2 ≥ 9.6 - < 11.8, Q3 ≥ 11.8 - < 15.3, Q4 ≥ 15.3. All data represent the percentage of subjects in the overall population (%); P values represent Cochran-Armitage trend tests and error bars represent 95% confidence intervals
Subjects (%) stratified in no AKI/transient AKI and persistent AKI subgroups in ordinal groups of PaO2/FiO2 ratio. PaO2/FiO2 ratio: Q1 < 85, Q2 ≥ 85 - < 114, Q3 ≥ 114 - < 157, Q4 ≥ 157. All data represent the percentage of subjects in the overall population (%). P values represent Cochran-Armitage trend tests, and error bars represent 95% confidence intervals
To assess whether standardized MV [5], as an index of the ventilatory load needed to keep PaCO2 within the normal range, may independently affect AKI incidence and persistence in severe COVID-19 patients, we performed an ordered multivariable analysis. After adjusting for confounders, including non-respiratory SOFA score, bicarbonate level at ICU admission, prone position, and admission center, standardized MV remained independently associated with a higher risk to develop AKI and AKI persistence (Table 3).
Hemodynamic parameters
We examined mean arterial pressure, heart rate, central venous pressure, vasopressor requirement, and lactate levels during the first ICU week in our population to evaluate the relationship between hemodynamics and renal function. A trend toward a more severe hemodynamic impairment was observed in patients with AKI. Patients with persistent AKI presented higher lactates, heart rate, and cardiovascular SOFA scores. No difference was reported in mean arterial pressure and central venous pressure among the groups (Table 2).
Outcomes
A similar incidence of thromboembolic complications (i.e., stroke, venous thromboembolism (VTE), pulmonary thromboembolism (PTE)) among the groups was found. Patients without AKI show a higher rate of bacterial over-infection during their ICU stay. Patients with persistent AKI show shorter duration of both invasive and non-invasive ventilation and shorter ICU and hospital length of stay. A significant tendency to higher ICU mortality was observed by moving through the AKI spectrum (Table 2). No difference in renal recovery was described when comparing transient AKI and persistent AKI classes (Table 2).
Discussion
The main results of this study can be summarized as follows:
-
Among 157 COVID-19 patients on mechanical ventilation, 47% developed AKI: 10% had transient AKI, and 37% had persistent AKI.
-
Across increasing severity of AKI groups, despite similar levels of paCO2, we observed an increased minute ventilation and a higher standardized minute ventilation, a robust proxy of dead space.
-
The degree of hypoxia was not associated with differences in AKI severity.
-
After adjusting for other clinical and laboratory covariates, standardized minute ventilation remained an independent predictor of AKI development and persistence.
-
d-dimer levels were increased early after ICU admission only in the persistent AKI group.
Among mechanically ventilated COVID-19 patients admitted to the ICU, AKI is a common yet severe complication. In our cohort, we observed a 46.5% incidence of AKI. Similar results were found in a recent study testing AKI incidence in patients with severe COVID-19, they observed a 53% AKI incidence in their patients [7]. In severe COVID-19 patients, a higher AKI incidence than in “typical” ARDS has been reported [3, 4]. Independent risk factors for AKI in non-COVID-19 ARDS include sepsis, non-cardiogenic shock, transfusion-related acute lung injury, and pancreatitis [31, 32], which were absent or extremely rare in our population. Therefore, other factors must be examined as potential causes of AKI in our cohort. We acknowledge that in emergency settings, such as during the initial European wave of COVID-19, the impracticality of maintaining a high standard of care may lead to severe respiratory distress, dehydration, and hypovolemia, potentially resulting in more severe kidney failure. Patients with persistent AKI showed to have higher serum creatinine and urea at ICU admission and a more positive fluid balance during the first 24 h, underling the severity of the organ failure in this group. Furthermore, in the persistent AKI group, we observed a higher percentage of patients with KDIGO stage 3 AKI, which has been associated with an increased risk of mortality in a COVID-19-related ARDS [29]. The rapid shortage of ICU beds was particularly important in small centers [6]. The surge of patients admitted to the hospital and the lack of ICU beds may be the reason for a higher incidence of AKI in Zingonia Hospital, a peripheric center very close to the Italian pandemic epicenter when compared with Monza and Galway hospitals.
We decided to stratify our patients according to the ADQI definition of transient and persistent AKI [24], considering the clinical relevance of this recent classification. When considering our cohort of AKI patients, 10 (21%) subjects developed transient AKI, while 58 (79%) had persistent AKI. In a retrospective analysis of all-cause ICU patients, the incidence of transient and persistent AKI was 73.4 and 16.5%, respectively [33]. Conversely, in septic patients, the incidence of transient and persistent AKI was 18.4% and 81.6%, and sustained AKI was found to be independently associated with sepsis mortality, as well as inflammatory and procoagulant responses [34]. In our study, COVID-19-related AKI in ICU was frequent and persistent. This finding is consistent with previous literature [35] and may suggest some overlap** mechanisms with sepsis-related AKI. We observed a significant trend toward higher ICU mortality in the persistent AKI group, in line with a previous report, where persistent AKI has been correlated with higher progression to CKD, morbidity, and mortality [36].
When analyzing inflammatory biomarkers, white blood cell count at ICU admission showed a higher trend in persistent AKI patients at ICU admission and during the first 3 days of ICU. Despite similar serum procalcitonin (PCT) at ICU admission, higher PCT levels were found during the first ICU week. Prolonged persistence of plasma PCT should include both the possibility of a sustained production and an impaired renal clearance of procalcitonin in the context of AKI [37]. Unexpectedly, a lower rate of bacterial over-infection was observed in the two AKI groups compared with the patients without AKI, suggesting that the sustained inflammation and its possible role in AKI persistence may not be due to bacterial over-infection.
A sustained inflammatory response and consequent coagulation activation may play a role in the development and persistence of COVID-19-related AKI. The SARS-Cov2 virus causes a dysregulation of the immune response leading to cytokine storm and macrophage activation syndrome. In this context, the complement activation stimulates coagulation pathways [38]. Inflammation and coagulation stimulate each other and may expose the kidney to intensive and repeated stimuli. Furthermore, glomerular and peritubular capillaries obstruction, due to erythrocyte aggregation and fibrin thrombi, has suggested a role of kidney thrombotic microangiopathy in COVID-19-related AKI pathophysiology [20, 21]. The significant effect of the AKI group on d-dimer, along with a higher level of d-dimer observed in the persistent AKI group when compared with the no AKI group on the first day of ICU may support this pathological mechanism in our population.
Despite no difference in the severity of hypoxemic respiratory failure (i.e., similar PaO2/FiO2 ratio and respiratory system mechanics at ICU admission) among the subgroups, an increased need for higher MV at ICU admission was required through the AKI spectrum. Furthermore, a higher MV was also required in the persistent AKI cohort during the following days until day 7. When considering the average respiratory parameters during the first week of ICU, we observed a significant trend towards a lower EtCO2 and a higher estimated dead space among the three groups. We noted a significantly higher percentage of subjects develo** persistent AKI in the higher MV and standardized MV quartiles with a trend toward a higher percentage of persistent AKI in the quartiles with more hypercapnic patients. Sustained hypercapnia was not associated with an increase in serum creatinine in non-COVID-19 ARDS [39]. However, our findings are consistent with previous reports about critically ill COVID-19 and ARDS patients, where an increased dead space [40, 41] and a significant impairment in CO2 clearance [39] were described.
We were not able to support the perfusion deficit as the leading cause of increased dead space through autopsy evaluations or imaging techniques. However, previous autopsy data reported inflammatory infiltration of endothelial cells and micro-thrombosis in the lung tissue of COVID-19 patients [42].
In our cohort, standardized MV is independently associated with the onset and persistence of AKI after adjusting for confounding factors. Although standardized MV does not directly measure alveolar dead space, it can be considered a strong proxy of dead space [30], as it effectively characterizes the additional ventilatory load required to maintain PaCO2 within the normal range. An increase in standardized MV has been observed with the severity of ARDS [5].
The absence of association between bicarbonate with AKI in the multivariable model suggests that the increase in the MV is probably aimed at kee** acceptable CO2 levels because of higher dead space in the presence of AKI. Even if our hypothesis needs to be further investigated from a histologic and imaging point of view, our preliminary findings suggest that a perfusion deficit may be a common mechanism in the development of lung and kidney failure.
The proportion of patients who underwent prone positioning decreased from the no-AKI to the persistent AKI group. Furthermore, patients who underwent prone positioning were ventilated with higher PEEP levels, than those who did not, which may be explained by a higher severity of respiratory failure in patients undergoing prone position. The benefits related to a more homogeneous ventilation and distribution of total stress and strain, which leads to a reduced risk of VILI [ The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Acute kidney injury Alanine transaminase Activated partial thromboplastin time Acute respiratory distress syndrome Aminotransferase Body mass index Chronic kidney disease Chronic obstructive pulmonary disease C-reactive protein Continuous renal replacement therapy Central venous pressure Compliance of respiratory system Driving pressure End-tidal CO2 Inspiratory fraction of oxygen Heart rate Ideal body weight Intensive care unit Intensive care unit length of stay Invasive mechanical ventilation Inhaled nitric oxide International normalized ratio Kidney Disease Improving Global Outcomes Lactate dehydrogenase Mean arterial pressure Minute ventilation Non-invasive mechanical ventilation Obstructive sleep apnea syndrome Arterial partial pressure of carbon dioxide Arterial partial pressure of oxygen Procalcitonin Positive end-expiratory pressure Pulmonary thromboembolism Respiratory rate Renal replacement therapy Sequential organ failure assessment Tidal volume Ventilator-free days Venous thromboembolism Veno-venous extracorporeal membrane oxygenation White blood cells Hoste EA, Bagshaw SM, Bellomo R et al (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41(8):1411–1423. https://doi.org/10.1007/s00134-015-3934-7 Ostermann M, Chang RW (2011) Impact of different types of organ failure on outcome in intensive care unit patients with acute kidney injury. J Crit Care 26(6):635.e1–635.e10. https://doi.org/10.1016/j.jcrc.2011.05.014 Darmon M, Clec’h C, Adrie C et al (2014) Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol 9(8):1347–1353. https://doi.org/10.2215/CJN.08300813 McNicholas BA, Rezoagli E, Pham T et al (2019) Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit Care Med 47(9):12161225. https://doi.org/10.1097/CCM.0000000000003832 Bellani G, Laffey JG, Pham T et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries [published correction appears in JAMA. 2016 Jul 19;316(3):350] [published correction appears in JAMA. 2016 Jul 19;316(3):350]. JAMA 315(8):788–800. https://doi.org/10.1001/jama.2016.0291 Grasselli G, Pesenti A, Cecconi M (2020) Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 323(16):1545–1546. https://doi.org/10.1001/jama.2020.4031 Fabrizi F, Alfieri CM, Cerutti R, Lunghi G, Messa P (2020) COVID-19 and acute kidney injury: a systematic review and meta-analysis. Pathogens 9(12):1052. https://doi.org/10.3390/pathogens9121052. Published 2020 Dec 15 Yang X, Yu Y, Xu J et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26]. Lancet Respir Med 8(5):475–481. https://doi.org/10.1016/S2213-2600(20)30079-5 Murdaugh HV Jr, Sieker HO, Manfredi F (1959) Effect of altered intrathoracic pressure on renal hemodynamics, electrolyte excretion and water clearance. J Clin Invest 38(5):834–842. https://doi.org/10.1172/JCI103865 Hall SV, Johnson EE, Hedley-Whyte J (1974) Renal hemodynamics and function with continuous positive-pressure ventilation in dogs. Anesthesiology 41(5):452–461. https://doi.org/10.1097/00000542-197411000-00009 Pesenti A, Slobod D, Magder S (2023) The forgotten relevance of central venous pressure monitoring. Intensive Care Med 49(7):868–870. https://doi.org/10.1007/s00134-023-07101-z Fogagnolo A, Grasso S, Dres M et al (2022) Focus on renal blood flow in mechanically ventilated patients with SARS-CoV-2: a prospective pilot study. J Clin Monit Comput 36(1):161–167. https://doi.org/10.1007/s10877-020-00633-5 Ottolina D, Zazzeron L, Trevisi L et al (2022) Acute kidney injury (AKI) in patients with COVID-19 infection is associated with ventilatory management with elevated positive end-expiratory pressure (PEEP). J Nephrol 35(1):99–111. https://doi.org/10.1007/s40620-02101100-3 Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D (2020) COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 201(10):1299–1300. https://doi.org/10.1164/rccm.202003-0817LE Darmon M, Schortgen F, Leon R et al (2009) Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intensive Care Med 35(6):1031–1038. https://doi.org/10.1007/s00134-008-1372-5 Liu KD, Glidden DV, Eisner MD et al (2007) Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 35(12):2755–2761 Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342(18):1301–1308. https://doi.org/10.1056/NEJM200005043421801 Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/path.1570 Zaim S, Chong JH, Sankaranarayanan V, Harky A (2020) COVID-19 and multiorgan response. Curr Probl Cardiol 45(8):100618. https://doi.org/10.1016/j.cpcardiol.2020.100618 Duarte-Neto AN, Monteiro RAA, da Silva LFF et al (2020) Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology 77(2):186–197. https://doi.org/10.1111/his.14160 Su H, Yang M, Wan C et al (2020) Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98(1):219–227. https://doi.org/10.1016/j.kint.2020.04.003 Klok FA, Kruip MJHA, van der Meer NJM et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147. https://doi.org/10.1016/j.thromres.2020.04.013 Grasselli G, Tonetti T, Protti A et al (2020) Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 8(12):1201–1208. https://doi.org/10.1016/S2213-2600(20)30370-2 Chawla LS, Bellomo R, Bihorac A et al (2017) Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13(4):241–257. https://doi.org/10.1038/nrneph.2017.2 Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138 Hirsch JS, Ng JH, Ross DW et al (2020) Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98(1):209–218. https://doi.org/10.1016/j.kint.2020.05.006 Gupta S, Hayek SS, Wang W et al (2020) Factors associated with death in critically ill patients with coronavirus disease 2019 in the US [published correction appears in JAMA Intern Med. 2020 Nov 1;180(11):1555] [published correction appears in JAMA Intern Med. 2021 Aug 1;181(8):1144]. JAMA Intern Med 180(11):1436–1447. https://doi.org/10.1001/jamainternmed.2020.3596 Inker LA, Eneanya ND, Coresh J et al (2021) New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 385(19):1737–1749. https://doi.org/10.1056/NEJMoa2102953 Rezoagli E, McNicholas BA, Madotto F et al (2022) Presence of comorbidities alters management and worsens outcome of patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Ann Intensive Care 12(1):42. https://doi.org/10.1186/s13613-022-01015-7. Published 2022 May 21 Rezoagli E, Laffey JG, Bellani G (2022) Monitoring lung injury severity and ventilation intensity during mechanical ventilation. Semin Respir Crit Care Med 43(3):346–368. https://doi.org/10.1055/s-0042-1748917 Rezoagli E, McNicholas B, Pham T, Bellani G, Laffey JG (2020) Lung-kidney cross-talk in the critically ill: insights from the Lung Safe study. Intensive Care Med 46(5):1072–1073. https://doi.org/10.1007/s00134-020-05962-2 McNicholas BA, Rezoagli E, Simpkin AJ, et al (2023) Correction : Epidemiology and outcomes of early-onset AKI in COVID-19-related ARDS in comparison with non-COVID-19-related ARDS: insights from two prospective global cohort studies. Crit Care 27(1):202. https://doi.org/10.1186/s13054-023-04487-6. Published 2023 May 26 Mo S, Bjelland TW, Nilsen TIL, Klepstad P (2022) Acute kidney injury in intensive care patients: Incidence, time course, and risk factors. Acta Anaesthesiol Scand 66(8):961–968. https://doi.org/10.1111/aas.14100 Uhel F, Peters-Sengers H, Falahi F et al (2020) Mortality and host response aberrations associated with transient and persistent acute kidney injury in critically ill patients with sepsis: a prospective cohort study. Intensive Care Med 46(8):1576–1589. https://doi.org/10.1007/s00134-020-06119-x Rubin S, Orieux A, Prevel R et al (2020) Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J 13(3):354–361. https://doi.org/10.1093/ckj/sfaa099. Published 2020 Jun 6 Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS (2017) Recovery after acute kidney injury. Am J Respir Crit Care Med 195(6):784–791. https://doi.org/10.1164/rccm.201604-0799OC Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K (2001) The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol 18(2):79–87. https://doi.org/10.1046/j.0265-0215.2000.00783.x Tuculeanu G, Barbu EC, Lazar M et al (2023) Coagulation disorders in sepsis and COVID-19-two sides of the same coin? A review of inflammation-coagulation crosstalk in bacterial sepsis and COVID-19. J Clin Med 12(2):601. https://doi.org/10.3390/jcm12020601. Published 2023 Jan 11 Madotto F, Rezoagli E, McNicholas BA et al (2020) Patterns and impact of arterial CO2 management in patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Chest 158(5):1967–1982. https://doi.org/10.1016/j.chest.2020.05.605 Graf J, Pérez R, López R (2022) Increased respiratory dead space could associate with coagulation activation and poor outcomes in COVID-19 ARDS. J Crit Care 71:154095. https://doi.org/10.1016/j.jcrc.2022.154095 Vasques F, Sanderson B, Formenti F, Shankar-Hari M, Camporota L (2020) Physiological dead space ventilation, disease severity and outcome in ventilated patients with hypoxaemic respiratory failure due to coronavirus disease 2019. Intensive Care Med 46(11):2092–2093. https://doi.org/10.1007/s00134-020-06197-x Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5 **n Y, Cereda M, Hamedani H et al (2018) Unstable inflation causing injury. Insight from prone position and paired computed tomography scans. Am J Respir Crit Care Med 198(2):197–207. https://doi.org/10.1164/rccm.201708-1728OC Motta-Ribeiro GC, Hashimoto S, Winkler T et al (2018) Deterioration of regional lung strain and inflammation during early lung injury. Am J Respir Crit Care Med 198(7):891–902. https://doi.org/10.1164/rccm.201710-2038OC Rezoagli E, Bellani G (2019) How I set up positive end-expiratory pressure: evidence- and physiology-based! Crit Care 23(1):412. https://doi.org/10.1186/s13054-019-2695-z. Published 2019 Dec 16 Rezoagli E, Bastia L, Brochard L, Bellani G (2023) Physical manoeuvres in patients with ARDS and low compliance: bedside approaches to detect lung hyperinflation and optimise mechanical ventilation. Eur Respir J 61(5):2202169. https://doi.org/10.1183/13993003.02169-2022. Published 2023 May 18 Dougal A, Rezoagli E, Pham T et al (2020) Patterns of use of adjunctive therapies in patients with early moderate to severe ARDS: insights from the LUNG SAFE study. Chest 157(6):1497–1505. https://doi.org/10.1016/j.chest.2020.01.041 Fogagnolo A, Grasso S, Morelli E et al (2024) Impact of positive end-expiratory pressure on renal resistive index in mechanical ventilated patients. J Clin Monit Comput. https://doi.org/10.1007/s10877-024-01172-z. Published online May 21 Montomoli J, Rezoagli E, Bellini V, Finazzi S, Bignami EG (2023) A "generalized wayfinding" paradigm for improving AKI understanding and classification: insights from the Dutch registries. Minerva Anestesiol 89(7-8):724–726. https://doi.org/10.23736/S0375-9393.23.17256-7. in the manuscript flow Yoon SY, Kim JS, Jeong KH, Kim SK (2022) Acute kidney injury: biomarker-guided diagnosis and management. Medicina (Kaunas) 58(3):340. https://doi.org/10.3390/medicina58030340. Published 2022 Feb 23 The authors thank all the colleagues and healthcare providers involved in the critical care of COVID-19 patients in the participating centers.
Study collaborators:
Aine O’Connor, from the Department of Anesthesia and Intensive Care Medicine, Galway University Hospitals; Giacomo Bellani, Marco Giani and Matteo Pozzi, from the School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy and Department of Emergency and Intensive Care, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy; Silvia Sordi and Ilaria Alice Crippa, from the Department of Anesthesia and Intensive Care Medicine, Policlinico San Marco, Gruppo Ospedaliero San Donato, Zingonia, Bergamo, Italy. This work was supported by Institutional funding. AN: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft. BMN: Conceptualization; Data curation; Investigation; Methodology; Resources; Writing – review & editing. AM: Data curation; Investigation; Resources; Software; Writing – review & editing. ML: Data curation; Investigation; Writing – review & editing. VZ: Data curation; Investigation; Writing – review & editing. IM: Data curation; Investigation; Writing – review & editing. MA: Data curation; Investigation; Writing – review & editing. MG: Data curation; Investigation; Writing – review & editing. GV: Data curation; Investigation; Writing – review & editing. RR: Formal analysis; Investigation; Methodology; Writing – review & editing. GF: Funding acquisition; Investigation; Resources; Writing – review & Editing. JL: Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing – review & editing. ER: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Visualisation; Project administration; Supervision; Writing – original draft. This research was part of the STORM Study approved by Istituto Nazionale Malattie Infettive Lazzaro Spallanzani, Rome, Italy (Resolution no. 84/2020; NCT04424992). The local ethics committee of Galway University Hospitals, Galway (C.A. 2384), and Policlinico San Marco, Zingonia (Reg. Sperim. N. 118/20) approved the study. Informed consent was waived considering the observational, non-interventional nature of the study. Not applicable. The authors declare no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Additional file 1: Table S1. PEEP at ICU admission in patients who underwent prone position and who did not, stratified by AKI groups. Table S2. PEEP (average during the first ICU week) in patients who underwent prone position and who did not, stratified by AKI groups. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Nova, A., McNicholas, B., Magliocca, A. et al. Perfusion deficits may underlie lung and kidney injury in severe COVID-19 disease: insights from a multicenter international cohort study.
J Anesth Analg Crit Care 4, 40 (2024). https://doi.org/10.1186/s44158-024-00175-1 Received: Accepted: Published: DOI: https://doi.org/10.1186/s44158-024-00175-1Availability of data and materials
Abbreviations
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Consortia
Contributions
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Additional information
Publisher’s Note
Supplementary Information
44158_2024_175_MOESM1_ESM.docx
Rights and permissions
About this article
Cite this article
Keywords