Abstract
Background
Cyclic fluctuation levels of hormones in females during different phases of menstruation can lead to many favorable and unfavorable changes. Different researchers had investigated these changes and suggested that such hormonal fluctuations may lead to alterations in auditory functions indirectly. The evidence from different studies suggested variations in thresholds of female participants between pre-menstruation and post menstruation stages. However, to our best knowledge, no attempts have been made to assess the differential sensitivity in females across the four phases of the menstrual cycle. The present study aims to investigate these variations in the auditory system across the four phases of menstruation.
Methods and materials
The participants were 27 volunteers with the age range of 18–30 years, consisting of 12 females (experimental group) and 15 males (control group). Three psychoacoustics measures, i.e., differential limen of intensity (DLI), differential limen of frequency (DLF), and differential limen of time (DLT), to assess the differential sensitivity were performed to analyze any audiological changes that may occur during each menstrual cycle phase.
Results
The results of the study showed that the scores were significantly poorer (p < 0.05) in the menstrual phase compared to other phases for all the tests in females. The scores were significantly better (p > 0.05) at the premenstrual phase for all the tests in females. There was no significant difference (p > 0.05) in scores across the phases for all the tests in males. The results of the study agree with previous studies which also report the effect of hormonal changes during the menstrual cycle in various audiological tests.
Conclusion
The results of the current study support to the theory that changes in sex hormone levels at different phases of menstrual cycle can affect differential sensitivity.
Similar content being viewed by others
Background
Hormones play an essential role in biological and physiological expressions in the human body. Its systematical circulation throughout the various organs regulates the normal functioning of various systems and subsystems in both males and females. However, these hormones are present in both males and females; their levels may vary which is an important factor responsible for sex-based differences. This also contributes to the physiological differences between both genders and when this hormone fluctuates; it alters the normal functioning of various systems in the body. However, the length of menstrual cycles varies, but the average time frame is of 27–28 days, which has been divided into four phases: the menstrual phase (days 1–3), follicular phase (days 11–14), ovulation phase (days 17–22), and luteal phase (days 25–27). These fluctuations also include the auditory system which is a system responsible for hearing.
Evidence from the literature shows that the hormonal fluctuations during different phases of the menstrual cycle alter auditory functions which result in variation of auditory threshold throughout these phases [8]. Fluctuations of specific hormones during these phases have their effects on the physiological, behavioral, and emotional response of the body [13, 23].
During the menstrual phase, the levels of estrogen and progesterone are low, whereas a follicular phase is characterized by the rise in secretion of estrogen hormone. During ovulation phase, with the release of ovule, estrogen approaches its peak level just before ovulation. However, in the luteal phase, progesterone reaches its peak, while estrogen continues to drop. Most of the changes in females occur in the luteal phase. These changes include retention of fluids, weight gain, increased energy demands, changes in uptake of glucose, reduced gastrointestinal transit time, and sodium retention and the resulting endolymph hypertension [1, 3, 13, 18, 30].
There is ample of evidence in the literature that supports the notion of the auditory system being affected by these changes during the different phases of menstruation. Previous electrophysiological study had suggested that the normal cyclic variation of these sex hormones has its effects on the auditory pathways [31].
Another study suggests that higher estrogen levels fine-tune the auditory system’s ability to process speech and psychoacoustic phenomenon like temporal processing [27]. However, few studies have also revealed that the different phases of the menstrual cycle do not have any effect on hearing acuity [3, 9, 12].
Psychoacoustical measures are widely used in the field of audiology to assess the hearing acuity level as well as to understand and evaluate the functioning of different auditory processes taking place from the peripheral to the central auditory system. However, to our best knowledge, no attempts have been made to assess the differential sensitivity in females across the four phases of the menstrual cycle. The present study aims to investigate the variations in the auditory system across four phases of menstruation with help of tests that assess the differential sensitivity perception. The specific objectives were to compare the scores of difference limen for frequency (DLI), difference limen for intensity (DLI), and difference limen for time (DLT) in both females (across four phases) and in males (similar time as that of the phases).
Method
Participants
This is a longitudinal study, examining how the menstrual cycle affects the hearing thresholds. The participants included were 27 volunteers with the age range of 18–30 years, consisting of 12 females (experimental group) and 15 males (control group).
Inclusion and exclusion criteria
Three questions were asked for the selection of participants: total days of the menstruation period as well as the time between cycles and average duration of the menstrual cycle. The study comprised women with a history of regular menstrual cycles (27–31 days) [7] and with the presence of no abnormal premenstrual tension symptoms such as dizziness, headaches, blurred vision, or extreme nausea. Females taking hormonal therapy, above the age of 30, were removed from the study due to the possibility of hormonal irregularities in that age range. Those with the history of hearing loss, ear discharge, and endocrine abnormalities were also excluded.
The participants were chosen using a random sampling procedure. Before the audiological examinations, the female volunteers were interviewed about their audiological background and menstrual cycle. The individuals were required to have no history of ear disease, use of steroids in the past, or diagnosed with premenstrual syndrome. Pure-tone audiometry was done to ensure that all of the patients had normal hearing, with hearing thresholds of less than 15 dBHL at octave frequencies between 250 and 8000 Hz. Immittance audiometry was administered to rule out any middle ear abnormality. All the participants exhibited “A”-type tympanogram, along with the presence of acoustic reflexes which indicated normal middle ear function. The population’s involvement in the study was entirely voluntary; they were not compensated for their time. Each participant signed an informed written consent form.
Compliance with Ethical Standards
All of the experiments in this study were performed on humans using noninvasive procedures, as directed by the institute’s Ethics Approval Committee. Before starting the study, permission was obtained from the Ethical Review Board of All India Institute of Speech and Hearing, and the ethical committee had approved the conduction of the study. The ethical number was also allotted by the committee, i.e., SH/IRB/2021–22/32. Before participating in the study, all of the procedures were explained to the participants, and they were requested to give their informed consent.
Instrumentation
A maximum likelihood procedure (MLP) toolbox designed in MATLAB (Version R2010a) software installed on a laptop was used to administer all the experiments. It uses the adaptive maximum-likelihood technique to estimate auditory thresholds quickly and accurately [15]. Stimuli were presented binaurally at an intensity of 60 dBHL using HDA-200 headphones. A sampling rate of 44,100 Hz was used to create MLP stimuli, and the threshold was tracked using three alternative forced choice (AFC) paradigms depending upon the experiment. A psychometric function with a 79.4% correct response criterion was used to obtain the thresholds. For each test, five to six practice items were given before starting the testing phase. All of the fundamental experiments were conducted in a room that is acoustically treated and had acceptable noise levels (ANSI S3.1.1991) (R2013).
Test procedure
Three psychoacoustics measures, i.e., DLI, DLF, and DLT, were used to assess the differential sensitivity. The data was collected over the course of 1 month, with the first session during the menstrual phase (days 1–3), second during the follicular phase (days 11–14), third during the ovulation phase (days 17–22), and fourth during the luteal phase (days 25–27) of the menstrual cycle.
Frequency discrimination
This test assesses the minimum frequency difference necessary to discriminate between two closely spaced frequencies. DLF was measured for a complex tone of 250 ms. Onset and offset of tones are gated on and off with two 10-ms raised cosine ramps. The frequency of the variable tone was varied adaptively, whereas the frequency of standard tones was kept constant [15]. The minimum and maximum frequency deviations used were 330.01 Hz and 390.01 Hz. On each trial of three blocks, two blocks with standard frequency of a complex tone and one with variable frequency, which is always higher than the standard frequency, were used. The variable block was identified by the participant for 30 trials to complete the test.
Intensity discrimination
The minimum intensity difference necessary to discriminate between two otherwise same sounds was assessed. DLI was measured for a 250-ms complex tone having onset and offset of 10 ms of rise and fall time [15]. The level of the variable tone was varied continuously, and the level of standard tone was kept constant. The minimum and maximum intensity deviations used were at − 29.99 and − 20 dB. On every trial of three blocks, two had complex tone of standard intensity and one with variable intensity assigned randomly, which is constantly higher than the standard intensity. The subject has to recognize the variable block for all 30 trials.
Duration discrimination
The procedure involves finding the minimum time difference necessary to discriminate between two otherwise same sounds. DLT was measured for a complex tone with the onset and offset gates of 10 ms [15]. The duration of variable tone was varied and kept constant for the standard tone. The minimum and maximum intensity deviations used were at 250.1 ms and 450.1 ms. Three blocks of every trial were presented with two blocks of complex tone of standard duration and one with variable duration, which is consistently longer than the standard duration. Across the 30 trials, the participant’s goal was to recognize the variable block.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 21, manufactured by International Business Machines Corporation (IBM), was used to analyze the data. To evaluate if the data were normally distributed, the Shapiro–Wilk test was used. The repeated measure ANOVA was used to compare the test scores among different phases of menstrual cycle in female and within identical timeline in male subjects. To establish the level of significance across all the four phases of menstrual cycle, the comparison was performed using Sidak post hoc test.
Results
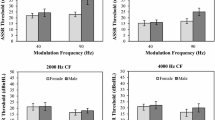
Shapiro–Wilk test of normality revealed that the data was normally distributed (p > 0.05). Mean and standard deviation were determined for all the tests in both males and females for all phases which are shown in Table 1. It shows that the scores were poorer during menstrual cycle and better in ovulation phase for females, whereas in males the scores were similar across phases for all the three tests.
Repeated measures ANOVA showed that the scores were significantly different across four phases in females for all three tests. Sidak post hoc test showed that the scores were significantly poorer (p < 0.05) in the menstrual phase compared to other phases for all the tests in females. The scores were significantly better (p < 0.05) at the ovulation phase for all the tests in females.
Repeated measures ANOVA test revealed that there was no significant difference (p > 0.05) in scores across the phases for all the tests in males. The results of repeated measures of ANOVA for all the three tests in both groups are shown in Table 2.
The mean and standard deviation of scores obtained for DLI, DLF, and DLT in males and females are shown in Figs. 1, 2, and 3, respectively. The findings of the present investigation are consistent with those of earlier studies, which also report the effect of hormonal changes during the menstrual cycle in various behavioral and electrophysiological tests.
Discussion
Previous literature investigating the effects of the menstrual cycle on auditory system has reported that the hearing thresholds become poorer during menstruation due to the withdrawal of sex hormones [4, 10]. Previous studies suggest changes in latencies of different auditory-based potentials across different phases of the menstrual cycle [20, 22]. There is still a lack of clarity on the association between the hormonal changes during the menstrual cycle and hearing issues. The findings of our study reveal that changes in hormonal secretion during the menstrual cycle has similar yet significant effects on the processes responsible for differential limen of intensity, frequency, and time, as evident by the similar results observed in all three tests for females. This test is measures of auditory functions involving temporal processes and is highly sensitive to any alteration in the auditory system [17, 19, 21, 29]. Due to the existence of estrogen and progesterone receptors in the cochlea, hormone variation during different phases of menstrual cycle causes the auditory system’s homeostasis to be disturbed and indirectly modulate auditory processes. The synaptic transmission at the level of the brainstem was impacted by these ovarian steroids, estrogen, and progesterone [2] which can affect these psychoacoustic measures. Similar results have been obtained for speech perception in noise, where increased levels of progesterone interfere with the identification of the speech, especially at the menstrual and luteal phases. The scores were highest in the ovulation phase, where there is a maximum secretion of estrogen [26]. Estrogen is known to excite the auditory nerve faster and results in better axon conduction leading to better auditory processing. It also increases the release of excitatory neurotransmitters, which leads to better auditory processing in ovulation phases compared to luteal and menstrual phases [26]. The absence of difference in scores for males strongly supports the effect of sex hormones on auditory processing abilities.
The physiological actions of estrogens are mediated through estrogen receptors (ERs), which are of two subtypes: alpha receptors (ERα) and beta receptors (ERβ) [5]. These receptors have been identified in the inner ear at the cochlear level including spiral ganglion type 1 cells, the stria vascularis, and cochlear blood vessels. The receptors indirectly influence auditory transmission, by affecting the cochlear blood flow and cochlear fluid electrolyte balance in the stria vascularis [2].
Recent advances and research in this area determined the presence of estrogen, and progesterone receptors in the human inner ear confirm the oto-protective role of estrogen [6, 30]. Studies have confirmed that those women who undergone hormonal therapy particularly for estrogen did not develop hearing loss in comparison with those who did not undergo hormonal therapy, and this is the reason why women develop hearing loss at an early age than men [16].
The results from present study confirm that the ovulation phase has the least effect on hearing sensitivity, while the other three phases have a negative effect resulting in poorer scores. The scores were significantly poorer for the menstruation phase; this can be due to the drop in secretion level of the estrogen [28]. During the follicular phase, the level of estrogen begins to rise which is characterized by the improvement in scores of the participants [24]. However, difference in the scores between first two phases is not statistically significant; there is a slight improvement in the scores during the follicular phase which again highlights the importance of this hormone in regulating the mechanism of hearing. The scores were significantly better in the ovulation phase as the estrogen level reaches its peak during this phase with the release of ovule [24]. Hence, the maximum improvement in scores during this phase confirms the oto-protective as well as the excitatory nature of this hormone. It maintains the homeostasis of hearing apparatus and facilitates the conduction of nerve impulses to higher centers.
The higher level of estrogen modulates the glutamate transmission which is an excitatory neurotransmitter, altering the speed of neurotransmission. The finding from the literature suggests that the higher level of estrogen also results in shorter ABR latencies [11]. An increase in the level of estrogen raises the level of neurosteroids which is responsible for the inhibition of gamma-aminobutyric acid (GABA), inhibitory in nature, and an antagonist to the glutamate. The luteal phase is characterized by the drop in the level of estrogen, while the progesterone level reaches its peak during this phase [32].
Progesterone has an inhibitory role in the central nervous system which helps to balance the excitatory effects of estrogen. The drop in the performance during this phase in the subjects of this study may be due to the combined effect of higher level of progesterone and lower level of estrogen during this phase. Elevated progesterone levels during the luteal phase may alter electrolyte fluid levels, which may result in ear fullness, imbalance, ringing sensation in ears, or symptoms similar to Meniere’s disease [14]. In contrast to estrogen, progesterone may modulate the GABA positively, increasing the GABA-binding neurosteroids and hence inhibiting the neuronal excitability [25].
Limitations of the study
Despite the fact that most aspects of the study were well controlled, the sample size was small due to methodological and logistical difficulties; the participants were required to complete repeated follow-ups at each phase, and many individuals did not attend all the sessions, thus reducing the sample size. Because the age group evaluated was constricted, a larger number of individuals could not be included. However, the current study’s finding was significant and justifies the need, including additional tests to determine differential sensitivity would have further reinforced the findings. The behavioral component of the tests, including the response pattern and method, was complex than the conventional ones, reducing the likelihood of receiving a correct response.
Conclusions
The results of the current study support to the theory that changes in sex hormone levels at different phases of menstrual cycle can affect differential sensitivity. Hence, the menstrual phase should be considered as an essential variable that should be controlled while carrying out the tests for differential sensitivity in females for clinical and research purposes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adriztina I, Adnan A, Adenin I, Haryuna SH, Sarumpaet S (2016) Influence of hormonal changes on audiologic examination in normal ovarian cycle females: an analytic study. Int Arch Otorhinolaryngol 20(4):294–299. https://doi.org/10.1055/s-0035-1566305
Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM (2008) Hormones and the auditory system: a review of physiology and pathophysiology. Neuroscience 153(4):881–900. https://doi.org/10.1016/j.neuroscience.2008.02.077
Arruda PO, Silva IMDC (2008) Estudo das emissões otoacústicas durante o ciclo hormonal feminino. Braz J Otorhinolaryngol 74(1):106–111. https://doi.org/10.1016/S1808-8694(15)30759-X
Baker M, Audiology, E. W.-B. J. of, & 1977, undefined (1977) Sex of listener and hormonal correlates of auditory thresholds. Taylor Francis 11(3):65–68. https://doi.org/10.3109/03005367709078835
Caruso S, Cianci A, Grasso D, Agnello C, Galvani F, Maiolino L, Serra A (2000) Auditory brainstem response in postmenopausal women treated with hormone replacement therapy: a pilot study. Menopause 7(3):178–183. https://doi.org/10.1097/00042192-200007030-00008
Charitidi K, Meltser I, Tahera Y, Canlon B (2009) Functional responses of estrogen receptors in the male and female auditory system. Hear Res 252(1–2):71–78. https://doi.org/10.1016/J.HEARES.2008.12.009
Cox JR (1980) Hormonal influence on auditory function. Ear Hear 1(4):219–222. https://doi.org/10.1097/00003446-198007000-00008
Da Silva Souza D, Luckwu B, De Andrade WTL, De Figueiredo Pessoa LS, Do Nascimento JA, Da Rosa MRD (2017) Variation in the hearing threshold in women during the menstrual cycle. Int Arch Otorhinolaryngol 21(4):323–328. https://doi.org/10.1055/s-0037-1598601
De Lima Resende LA, Silva MD, Impemba F, Achôa NB, Schelp AO (2000) Multimodal evoked potentials and the ovarian cycle in young ovulating women. Arq Neuropsiquiatr 58(2 B):418–423. https://doi.org/10.1590/s0004-282x2000000300004
Elkind-Hirsch KE, Malinak LR, Wallace E, Jerger JJ (1994) Sex hormones regulate ABR latency. Otolaryngology Head Neck Surg 110(1):46–52. https://doi.org/10.1177/019459989411000105
Emami SF, Gohari N, Ramezani H, Borzouei M (2018) Hearing performance in the follicular-luteal phase of the menstrual cycle. Int J Otolaryngol 2018:1–6. https://doi.org/10.1155/2018/7276359
Fagan PL, Church GT (1986) Effect of the menstrual cycle on the auditory brainstem response. Int J Audiol 25(6):321–328. https://doi.org/10.3109/00206098609078396
Farage MA, Neill S, MacLean AB (2009) Physiological changes associated with the menstrual cycle a review. Obstet Gynecol Surv 64(1):58–72. https://doi.org/10.1097/OGX.0b013e3181932a37
Galvani F, Maiolino L (2000) Auditory brainstem response in postmenopausal women treated with hormone replacement therapy: a pilot study. Researchgate.Net. https://doi.org/10.1097/00042192-200007030-00008
Grassi M, Soranzo A (2009) MLP: a MATLAB toolbox for rapid and reliable auditory threshold estimation. Behav Res Methods 41(1):20–28. https://doi.org/10.3758/BRM.41.1.20
Hederstierna C, Hultcrantz M, Collins A, Rosenhall U (2007) Hearing in women at menopause. Prevalence of hearing loss, audiometric configuration and relation to hormone replacement therapy. Acta Otolaryngol 127(2):149–155. https://doi.org/10.1080/00016480600794446
Henry FM (1948) Discrimination of the duration of a sound. J Exp Psychol 38(6):734–743. https://doi.org/10.1037/h0058552
Ishii C, Nishino LK, Herrerias de Campos CA (2009) Vestibular characterization in the menstrual cycle. Braz J Otorhinolaryngol 75(3):375–380. https://doi.org/10.1016/s1808-8694(15)30655-8
Köning E, Lüscher E (1962) Difference limen for intensity. Int J Audiol 1(2):198–202. https://doi.org/10.3109/05384916209074042
Mann, N., Sidhu, R., diagnostic, R. B.-J. of clinical and, & 2012, undefined (2012) Brainstem auditory evoked responses in different phases of menstrual cycle. Ncbi.Nlm.Nih.Gov. Retrieved January 13, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3552194/
Meurmann OH (1954) The difference limen of frequency in tests of auditory function. Acta Otolaryngol 43(S118):144–155. https://doi.org/10.3109/00016485409124004
Natarajan N, Dharshni KP, Ukkirapandian K, Lakshmi A (2014) Brain stem auditory evoked response during different phases of menstrual cycle. Researchgate.Net. 6. https://doi.org/10.5455/ijmsph.2014.010420143
Poromaa IS, Gingnell M (2014) Menstrual cycle influence on cognitive function and emotion processing—from a reproductive perspective. Front Neurosci 8:380. https://doi.org/10.3389/FNINS.2014.00380
Reed BG, Carr BR (2018) The normal menstrual cycle and the control of ovulation. Endotext. https://www.ncbi.nlm.nih.gov/books/NBK279054/
Rogawski MA (2003) Progesterone, neurosteroids, and the hormonal basis of catamenial epilepsy. Ann Neurol 53(3):288–291. https://doi.org/10.1002/ana.10534
Sao T, Hearing CJ, Communication, B. and, & 2016, undefined (2016) Effects of hormonal changes in temporal perception, speech perception in noise and auditory working memory in females. Taylor & Francis 14(2):94–100. https://doi.org/10.3109/21695717.2016.1155837
Sao T, Jain C (2016) Effects of hormonal changes in temporal perception, speech perception in noise and auditory working memory in females. Hear Bal Commun 14(2):94–100. https://doi.org/10.3109/21695717.2016.1155837
Simmen FA, Simmen RCM (2006) Orchestrating the menstrual cycle: discerning the music from the noise. Endocrinology 147(3):1094–1096. https://doi.org/10.1210/en.2005-1451
Sreedhar A, Prakash P, Umashankar A, Prabhu P (2021) Effect of efferent stimulation on the differential sensitivity in individuals with normal hearing. Indian J Otolaryngol Head Neck Surg. https://doi.org/10.1007/S12070-021-02852-X
Stenberg AE, Wang H, Fish J, Schrott-Fischer A, Sahlin L, Hultcrantz M (2001) Estrogen receptors in the normal adult and develo** human inner ear and in Turner’s syndrome. Hear Res 157(1–2):87–92. https://doi.org/10.1016/S0378-5955(01)00280-5
Yadav A, Tandon OP, Vaney N (2002) Auditory evoked responses during different phases of menstrual cycle. Indian J Physiol Pharmacol 46(4):449–456
Ziomkiewicz A, Pawlowski B, Ellison PT, Lipson SF, Thune I, Jasienska G (2012) Higher luteal progesterone is associated with low levels of premenstrual aggressive behavior and fatigue. Biol Psychol 91(3):376–382. https://doi.org/10.1016/j.biopsycho.2012.08.001
Acknowledgements
The authors acknowledge with gratitude Prof. M. Pushpavathi, Director, All India Institute of Speech and Hearing, Mysore, for permitting to introduce this concept being a part of the institute.
Funding
There is no funding by any agency for the manuscript.
Author information
Authors and Affiliations
Contributions
The authors certify that they qualify for authorship according to the below mentioned contribution to the manuscript: SK was involved in conception, design, data collection and processing, and literature review and writing. SY was involved in conception, design, data collection and processing, and literature review and writing. PP was involved in conception, design, supervision, data analysis, and interpretation and critical review. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Before starting the study, permission was obtained from the Ethical Review Board of All India Institute of Speech and Hearing, and the ethical committee had approved the conduction of the study. The ethical number was also allotted by the committee, i.e., SH/IRB/2021–22/32. Before participating in the study, all of the procedures were explained to the participants, and they were requested to give their informed consent.
The manuscript adheres to the ethical standards according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kasera, S., Yadav, S. & Prabhu, P. Effect of hormonal changes during menstrual cycle on measures of differential sensitivity: a cross-sectional study. Egypt J Otolaryngol 39, 60 (2023). https://doi.org/10.1186/s43163-023-00421-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-023-00421-3