Abstract
Background
Where there is no identifiable cause of otologic symptoms like hearing loss and tinnitus, it is believed that etiology could possibly involve a vascular loop in AICA compressing the vestibulo-cochlear nerve within the internal auditory canal. In this study, we aim to evaluate whether there is any association of AICA vascular loops with unexplained tinnitus.
Methods
The present prospective study was conducted in the Department of ENT, SMGS Hospital, GMC Jammu, from October 2020 to March 2022 on 131 subjects with unexplained tinnitus. All subjects were subjected to contrast enhanced MRI brain (3D fast spin echo T2W1 with drive equilibrium pulse) for evaluation of AICA using Chavda classification—type 1 (lying in CP angle, but not entering IAC), type 2 (entering IAC but extending < 50% of length of IAC), and type 3 (extending > 50% of length of IAC).
Results
Out of 131 unexplained tinnitus cases, 76 patients (58%) had AICA loop on MRI. Out of 16 patients with right sided tinnitus, 11 patients had AICA on same side, while 5 patients had AICA on the opposite (left) side. Out of 26 patients with left-sided tinnitus, 12 patients had AICA on same side, while 14 patients had AICA on the opposite (right) side, these findings being statistically insignificant (p = 0.153).
Conclusion
From our study, we can conclude that the presence of AICA vascular loop either on CP angle or within IAC in tinnitus patients is an incidental finding and has no role in its etiology.
Similar content being viewed by others
Background
Cerebellopontine fissure is made when the cerebellum folds over pons, creating a sharp angle between the two called as cerebellopontine angle. The cerebellopontine angle is an important region in the brain as the trigeminal nerve, facial nerve, and vestibulocochlear nerve exit the brain at this level. Besides, these cranial nerves—CSF, arachnoid tissue, and anteroinferior cerebellar artery (AICA)—form other important contents of the CP angle [1].
AICA is a branch of basilar artery and gives off a labyrinthine artery that enters the internal auditory canal, along with the facial nerve, vestibulocochlear nerve, and nervus intermedius. In some 20–40% cases, a loop of AICA also enters IAC. Hence, due to its anatomical course, AICA is commonly involved in various compression syndromes [2].
The term vascular compression syndrome refers to the group of diseases caused by mechanical irritation of cranial nerve by other tissues, usually a blood vessel. This term was given by McKenzie in 1936 and popularized by Janetta in 1975 [3]. However, the first case possibly dates back to 1875 where a vertebral artery aneurysm was found to be a compressing facial nerve. The most commonly involved cranial nerves are the trigeminal nerve, facial nerve, and vestibulocochlear and glossopharyngeal nerve. The compression syndromes are usually of concern when they are symptomatic [4].
In some cases, where there is no identifiable cause of otologic symptoms like hearing loss and tinnitus, it is believed that etiology could possibly involve a vascular loop in AICA compressing the vestibulo-cochlear nerve within the internal auditory canal. But as such vascular loops are seen in asymptomatic individuals as well, there is an ongoing debate whether AICA loops are actually responsible for otological symptoms or not [5, 6].
Magnetic resonance imaging (MRI) is usually the radiological modality of choice to evaluate the temporal bone including the internal auditory canal and cerebellopontine angle in such patients with no identifiable cause of otological symptoms [7].
As there is very limited literature on this topic, especially in our local subpopulation, we, with our study, aim to fill these lacunae. In this study, we aim to evaluate whether there is any association of AICA vascular loops with unexplained tinnitus.
Methods
The present prospective study was conducted in the Department of ENT, SMGS Hospital, GMC Jammu, from October 2020 to March 2022 on 131 subjects with unexplained tinnitus.
The study was approved by the Institutional Ethics Committee. Written informed consent was taken from all subjects in the language they understood.
Relevant clinical history of hearing loss, tinnitus, and any other complaints was taken from all patients. General physical examination and systemic examination (CNS/CVS/respiratory) was done. Local ear examination, including otoscopy, was done. Examination of the nose, oral cavity, and indirect laryngoscopy was done.
All patients were subjected to pure tone audiometry. Pure tone average was calculated as average of 500, 1000, and 2000 Hz.
Routine blood investigations like CBC, RFT, LFT, triple serology (HIV, hepatitis B anti-HCV), blood sugar, thyroid profile, coagulation profile, VDRL, and ESR were done.
Inclusion criteria:
-
1)
Unexplained tinnitus
-
2)
Patients giving consent for participation in the study
Exclusion criteria:
-
1)
Metabolic/systemic/otologic causes of tinnitus
-
2)
History of ear surgery
-
3)
History of ear discharge
All subjects were subjected to contrast enhanced MRI brain (3D fast spin echo T2W1 with drive equilibrium pulse) for evaluation of AICA using Chavda classification—type 1 (lying in CPA, but not entering IAC), type 2 (entering IAC but extending < 50% of length of IAC), and type 3 (extending > 50% of length of IAC).
All data was entered in Microsoft Excel spreadsheet and analyzed and compared using the Statistical Package for Social Sciences (SPSS) software (version 21 for windows). Appropriate statistical analytical tests were applied as per the advice of statistician.
Results
Out of 131 unexplained tinnitus cases, 76 patients (58%) had AICA loop on MRI. In these 76 patients, the mean age of presentation in our study was 48.06 ± 14.79 years, with the majority of patients in the age group of 41–60 years (Table 1). Out of 60 patients, there were 36 male patients and 40 female patients, with female to male ratio being 1.11:1.
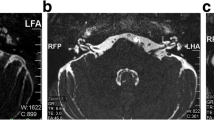
In our study out of 76 cases, 16 patients presented with tinnitus in the right ear and 26 patients in the left ear, and 34 patients had bilateral tinnitus (Table 2). In our study, out of 131 patients with unexplained tinnitus, 76 patients (58%) had AICA loop on MRI. Out of them, 46 cases had AICA type 2 loop (Fig. 1), 29 cases had AICA type 1 loop, and 1 case had AICA type 3 loop (Fig. 2).
In our study, out of 16 patients with right-sided tinnitus, 11 patients had AICA on the same side, while 5 patients had AICA on the opposite (left) side. Out of 26 patients with left-sided tinnitus, 12 patients had AICA on same side, while 14 patients had AICA on the opposite (right) side (Table 3), these findings being statistically insignificant (p = 0.153).
Discussion
The term “tinnitus” was introduced by Pliny and means “to ring.” Tinnitus can be defined as an auditory perception due to altered spontaneous activity within the auditory pathway, which arises as a result of aberrant excitation or inhibition. The underlying mechanism for occurrence of tinnitus can be abnormal afferent excitation at cochlear level, efferent dysfunction, and alteration of spontaneous activity with tonotopic reorganization [8].
Vascular compression syndromes are basically a clinical entity involving compression of one of the cranial nerves by a vessel. The pathophysiology of vascular compression syndromes could be impaired blood flow by a neurovascular compression or focal demyelination, reorganization, and axonal hyperactivity due to compression. However, although the pathophysiology of vascular compression syndromes can be applied on various conditions like hemifacial spasm and trigeminal neuralgia, it cannot fully explain audiovestibular symptoms like tinnitus [9].
It has been hypothesized that AICA loop can cause irritation of VIII cranial nerve, leading to gliosis, edema, axonal degeneration, and eventual fibrosis of the auditory nerve, leading to audiovestibular symptoms like tinnitus, vertigo, and hearing loss. These changes in the auditory nerve can also induce changes in the brainstem auditory nuclei. However, various radiological studies have shown nearly same incidence of AICA loops in the CP angle and IAC in symptomatic and asymptomatic cases, thus creating significant discrepancy regarding association of AICA loop and tinnitus [10, 11].
In our study, the mean age of presentation was 48.06 years, which was comparable to study conducted by Gultekin S et al. [12]; however, Bhatia A et al. [1] showed a younger mean age of presentation (35 years). In our study, more female subjects were seen as compared to males [statistically insignificant, p > 0.05)], similar to the study conducted by de Abreu Junior L et al. [13]. However, Kazawa N et al. [14] showed male preponderance in their study.
In our study, out of 131 patients with unexplained tinnitus, 76 patients (58%) had AICA loop on MRI. Out of them, 46 cases had AICA type 2 loop (Fig. 2), 29 cases had AICA type 1 loop, and 1 case had AICA type 3 loop (Fig. 1). Thus, according to our study, AICA type 2 was most commonly involved with tinnitus, followed by type 1 and type 3. Our finding was consistent with the study conducted by McDermott AL et al. [15] and de Abreu Junior L et al. [13]. However, studies conducted by Bhatia A et al. [1] and Kazawa N et al. [14] showed that AICA type 1 was most commonly associated with unexplained tinnitus. In contrast, Kim M et al. [16] suggested in their study that tinnitus was more frequent with AICA type 3.
In our study (Fig. 3), out of 16 patients with right-sided tinnitus, 11 patients had AICA on the same side, while 5 patients had AICA on the opposite (left) side. Out of 26 patients with left-sided tinnitus, 12 patients had AICA on the same side, while 14 patients had AICA on the opposite (right) side; these findings being statistically insignificant (p = 0.153). Similar finding was shown in their studies by Makins et al. [17], Gultekin et al. [12], and Grocoske et al. [18], who also concluded that there was no cause-effect relationship between tinnitus and vascular loops. However, Nowe et al. [19], Mc Dermott et al. [15], and Ryu et al. [20] suggested significant association between vascular loops in the internal auditory canal and tinnitus. Mc Dermott et al. [15] studied not only tinnitus but also the association of all cochleovestibular symptoms (including hearing loss) with AICA, hence concluding a significant association. We in our study did not include hearing loss/vertigo patients. Furthermore, Ryu et al. [20] explored posterior fossa in AICA patients for microvascular decompression and showed there was significant improvement in tinnitus postoperatively. We in our study did not operate to know the association between AICA and tinnitus but only focused on non-invasive methods like MRI and clinical findings. Again, Nowe et al. [20] grouped tinnitus as pulsatile and non-pulsatile to show significant association between vascular compression of the cisternal segment of eighth cranial nerve and non-pulsatile tinnitus, while stating pulsatile tinnitus was due to direct the transmission of pulsations to the cochlea due to a resonance effect in the petrous bone. We believed that grou** of tinnitus would not change our results.
Thus, in our study, the presence of AICA loop in patients with unexplained tinnitus could just be an incidental finding and AICA loops might be a normal anatomical variation, and thus, unnecessary microvascular decompression surgeries may not be performed. From our study, we could also conclude that the presence of vascular loop in IAC (AICA type 2 loop) does not always lead to reduced regional nerve vascular perfusion, and further research needs to be done on the complicated pathophysiology/symptomatology of microvascular syndromes.
Conclusion
From our study, we can conclude that the presence of AICA vascular loop either on CP angle or within IAC in tinnitus patients is an incidental finding and has no role in its etiology. Further research needs to be done on vascular loops to distinguish whether it is a normal variation or a pathological entity.
Availability of data and materials
Available with the corresponding author upon reasonable request
References
Bhatia A, Phukan P, Sharma B, Polley G (2021) Is there an association between anteroinferior cerebellar artery vascular loop and assymetrical hearing loss? Ann Indian Acad Otorhinolaryngol Head Neck Surg 5:2–7
Brackmann D, Crawford J, Green D (2006) Cerebellopontine angle tumors. Head and Neck Surgery- Otolaryngology, 4th edn. Lippincott Williams and Wilkins, Philadelphia, pp 2208–2230
Jannetta PJ (1975) Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Surg Forum 26:467–469
Shultze F (1875) Linksseitieer fatialiskrampf in folge cines aneurysma der aterid verteralis sinistra. Virchows Arch 65:385–391
Van der Steenstraten F, de Ru JA, Witkamp TD (2007) Is microvascular compression of the vestibulocochlear nerve a cause of unilateral hearing loss? Ann Otol Rhinol Laryngol 116:248–252
Herzog JA, Bailey S, Meyer J (1997) Vascular loops of the internal auditory canal. A diagnostic dilemma. Am J Otol 18:26–31
Kwan TL, Tang KW, Pak KK, Cheung JY (2004) Screening for vestibular schwannoma by magnetic resonance imaging: analysis of 1821 patients. Hong Kong Med J 10:38–43
Ceranic B, Luxon LM (2018) Tinnitus and other dysacuses. In: Gleeson M, Browning GG, Burton MJ et al (eds) Scott Brown’s Otorhinolaryngology, Head and neck surgery. Edward Arnold, Great Britain, pp 3594–3628
Nielsen VK (1984) Pathophysiology of hemifacial spasm. Ephaptic transmission an ectopic excitation. Neurology 34:418–426
Wilkins RH (1985) Neurovascular compression syndromes. Neurol Clin 3:359–372
Jung NY, Moon WJ, Lee MH, Chung EC (2007) Magnetic resonance cisternography: comparison between 3-dimensional driven equilibrium with sensitivity encoding and 3-dimensional balanced fast-field echo sequences with sensitivity encoding. J Comput Asssist Tomogr 31:588–591
Gultekin S, Celik H, Akpek S, oner Y, Gumus T, Tokgoz N. (2008) Vascular loops at the cerebellopontine angle: is there a correlation with tinnitus? AJNR Am J Neuroradiol 29:1746–1749
De Abreu JL, Kuniyoshi CH, Wolosker AB, Borri ML, Antunes A, Arashiro Ota VK et al (2016) Vascular loops in the anteroinferior cerebellar artey, as identified by magnetic resonance imaging and their relationship with otologic symptoms. Radiol Bras 49(5):300–304
Kazawa N, Togashi K, Ito J (2013) The anatomical classification of AICA/PICA branching and configurations in the cerebellopontine angle area on 3D drive thin slice T2W1 MRI. Clinical Imaging 37:865–870
McDermott AL, Dutt SN, Irving RM, Pahor AL, Chavda SV (2003) Anterior inferior cerebellar artery syndrome: fact or fiction. Clin Otolaryngol Allied Sci 28:75–80
Kim M, Chu H, Song MY, Lee SD, Kim HN (2012) The relationship between vestibulocochlear symptoms and types of the anterior inferior cerebellar artery (AICA) pathway in cerebellopontine angle. J Clin Otolaryngol 23:74–78
Makins AE, Nikolopoulos TP, Ludman C, O’Donoghue GM (1998) Is there a correlation between vascular loops and unilateral auditory symptoms? Laryngoscope 108:1739–1742
Grocoske FL, Mendes RD, Vosguerau R, Mocellin M, Oliveira MT, Koerner HN (2011) Neurotology findings in patients with diagnosis of vascular loop of cranial nerves VIII in magnetic resonance imaging. Intl Arch Otorhinolaryngol 15:418–425
Nowe V, de Ridder D, van de Heyning PH, Wang XL, Gieden J, van Goethem J et al (2004) Does the location of vascular loops in cerebellopontine angle explain pulsatile and non-pulsatile tinnitus. Eur Radiol 14:2282–2289
Ryu H, Yamamoto S, Sugiyama K, Nishizawa S, Nozue M (1999) Neurovascular compression syndrome of the eighth nerve- can the site of compression explain the symptoms? Acta Neurochir (Wien) 141:495–501
Acknowledgements
None
Funding
Nil
Author information
Authors and Affiliations
Contributions
AS and NG made contribution in design/writing of manuscript. MM and PK made contribution in data collection. AS made contribution in statistical analysis. All authors read and gave approval to the final version of submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after the approval by the Institutional Ethics Committee of Government Medical College, Jammu. Written informed consent was taken from all subjects or their legal guardian in case of age of patient being less than 18 years
Consent for publication
Written informed consent to publish patient’s clinical details was obtained from all subjects or their legal guardian in case of subjects under 18 years.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saraf, A., Gupta, N., Manhas, M. et al. Anterior inferior cerebellar artery loop and tinnitus—is there any association between them?. Egypt J Otolaryngol 38, 171 (2022). https://doi.org/10.1186/s43163-022-00369-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-022-00369-w